Abstract
BACKGROUND: The first step to the cure of Multiple myeloma (MM) is achieving a clinical complete response (CR). However, a majority of patients relapse in part due to the persistence of low levels of pathological plasma cells after treatment (minimal residual disease (MRD)). In previous work, we have shown that Next generation sequencing (NGS) of immunoglobulin (IG) rearranged genes is an effective technology to identify and quantify pathological clonal cells in MM, and also it is a good predictor of patient prognosis. However, this technology is a proprietary multiplex PCR, uses a reference plasmid, it is performed at the centralized laboratories, and it is not broadly available.
AIMS: To present a new in-house simplified method for quantification of IG gene clonal rearrangements by NGS. To test the suitability of the method for MRD detection in MM patients compared with 8-color high sensitivity Flow Cytometry (8c-MFC) data on a prospective way. To determine its capability as a predictor of patient prognosis.
METHOD: We use deep sequencing to prospectively analyzed DNA from bone marrow samples of 58 patients (58 diagnostic samples and 80 follow-up samples) of patients included the GEM10 clinical trial. The method employs the primers developed by BIOMED-2 for IGH and IGK, and a set of specific mathematical and bioinformatics tools. First, we identified the clonal rearrangement(s) (clonotype) in the diagnostic sample by fragment analysis and NGS. Second, we searched the NGS sequences from follow-up samples to detect and quantitate (MRD) the presence of the clonotype identified in the diagnostic sample. Overall Survival (OS) and Progression free Survival (PFS) were estimated by Kaplan-Meier survival analysis. The survival curves generated were compared with the log-rank test for trend.
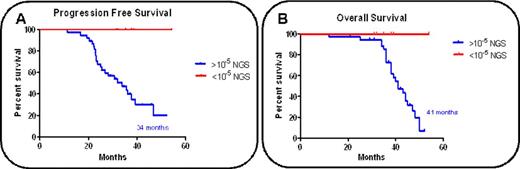
RESULTS: The sensitivity of the method is 10-5 for 150.000 cells. Reproducibility is >90%. The Spearman correlation coefficient between NGS and 8c-MFC data for diagnosis and follow-up samples was R=0.6903, p<0.0001. When we applied the criteria of 10-5 for NGS, discordant results were as follow: 121 cases were positive by both methods, 2 cases were positive (>10-5) by 8c-MFC but negative (<10-5) by NGS; 7 samples were negative (<10-5) by 8c-MFC but positive (>10-5) by NGS; and 7 cases were negative by both methods. Next we analyzed de correlation of OS and PFS with MRD levels determined by NGS and by 8c-MFC. For NGS data, median PFS (FigA) was 34 months for patients with values of MRD>10-5 vs not reached for patients with values of MRD<10-5 (hazard ratio [HR] = 3.69, P =0 .0109). Also, for 8c-MFC data, the patients with values of MRD<10-5 had longer PFS than those with values of MRD>10-5 (34 months versus 47; HR = 3.69, P =0 .0109). Median OS (FigB) was also significantly longer in MRD<10-5 (NGS) patients (not yet reached), compared to MRD>10-5 (NGS) patients (41 months, HR = 3.6, P =0.0495). For 8c-MFC we did not get a significant association (41 vs 50 months, HR=1.979, p=0.228). It should be noted that this comparison was made with the standard 8c-MFC and it is probable that the sensitivity will be inferior to that of the Next generation flow. There were no significant differences in terms of PFS between the data obtained by our in-house method, 8c-FCM and previous data obtained with LymphoSight technology.
CONCLUSIONS: Our data confirms that analysis of MRD levels determined by deep sequencing is capable of predict survival outcomes in myeloma patients. NGS sequencing of Ig genes is an effective technology to identify and quantify pathological clonal cells in MM. This approach is a methodological and economical alternative to MFC and other methods for MRD follow-up.
Paiva:Janssen: Consultancy; Celgene: Consultancy; BD Bioscience: Consultancy; Sanofi: Consultancy; Binding Site: Consultancy; Onyx: Consultancy; Millenium: Consultancy; EngMab AG: Research Funding. Ocio:Pharmamar: Consultancy, Research Funding; MSD: Research Funding; Novartis: Consultancy, Research Funding; Mundipharma: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy; Amgen/Onyx: Consultancy, Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Array BioPharma: Consultancy, Research Funding; Jassen: Honoraria. Mateos:Celgene: Consultancy, Honoraria; Takeda: Consultancy; Janssen-Cilag: Consultancy, Honoraria; Onyx: Consultancy. San Miguel:Novartis: Honoraria; Celgene: Honoraria; Janssen-Cilag: Honoraria; Bristol-Myers Squibb: Honoraria; Millennium: Honoraria; Onyx: Honoraria; Sanofi-Aventis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal