Abstract
Introduction: Several cooperative MM groups have shown that MRD monitoring may be relevant as biomarker to evaluate the efficacy of different treatment strategies, to support treatment decisions, and to act as surrogate for overall survival (OS) in MM. Because of its wider applicability, a significant fraction of available MRD data has been obtained using MFC that originally, was limited to 4- or 6-colors and measured a limited number of cells. It is assumed that the sensitivity of MFC can increase by usage of ≥8 markers and acquisition of greater cell numbers, but the degree of improved specificity and sensitivity remains unknown.
Methods: We aimed at determining the increment in specificity and sensitivity upon transition from first-generation 4-color into a second-generation 8-color MFC assay, by applying new computational tools developed by the EuroFlow consortium in elderly MM patients, enrolled in the GEM2010MAS65 study, for which MRD monitoring was performed with an 8-color monoclonal antibody combination - CD45-PacB/CD138-OC515/CD38-FITC/CD56-PE/CD27-PerCPCy5.5/CD19-PECy7/CD117-APC/CD81-APCH7 - and acquisition of ≥2x106 leukocytes (detection limit: 10-5). Time-to-progression (TTP) and OS were measured from diagnosis.
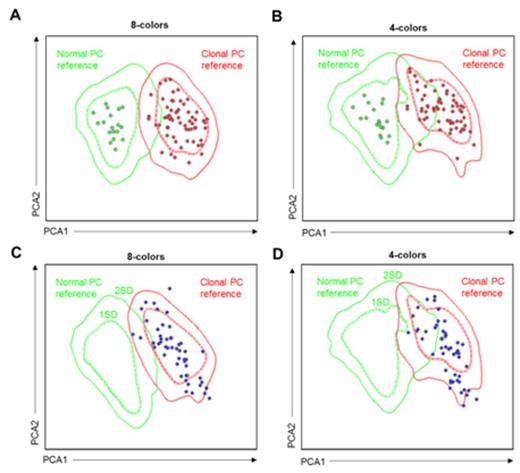
Results: First, we created a reference data file of normal (n=17) and clonal (n=71) plasma cells (PCs) derived from bone marrow samples of healthy individuals and MM patients (Figure 1A) in order to determine the individual contribution of each marker to the discrimination between normal vs. clonal PCs. Principal component analysis (PCA) showed that CD19 ranked as the most significant marker followed by CD56, CD81, CD27, CD117, CD45, forward scatter (FSC), CD38, CD138 and sideward scatter (SSC). Accordingly, the 8-color combination resulted in improved discrimination between normal vs. clonal PCs as compared to the former 4-color approach based only on CD38/CD56/CD19/CD45 (Figure 1B); in fact, CD81, CD27 and CD117 had higher independent value than CD45 in the PCA. Afterwards, we focused on 50 randomly selected MRD-positive patients enrolled in the GEM2010MAS65 study, to compare the performance of an 8- vs. 4-color software-guided classification of MRD cells. PCA based on 8-colors showed that all but two patients were accurately located in the clonal PC reference and outside 1 or 2 standard deviation (SD) curves of the normal PC reference (96% accuracy; Figure 1C); by contrast, using 4-color software-guided classification up to 9 patients became located in the overlapping area between 1 and 2 SD of the normal and clonal PCs references (82% accuracy; Figure 1D). Afterward, we investigated the increment in sensitivity due to the evaluation of 2x106 leukocytes with the second-generation 8-color flow assay instead of the standard 2x105 cells with the first-generation 4-color approach, by determining how many of the 50 MRD-positive patients would turn into MRD-negative if only 2x105 leukocytes had been analyzed (detection limit: 10-4). Interestingly, by reducing the number of visible events to 2x105, our results showed that up to 15 out of the 50 cases (30%) would become wrongly classified as MRD-negative. Then, we investigated the impact in TTP and OS of having MRD levels of 10-5 within a series of 163 patients enrolled in the GEM2010MAS65 and with MRD assessment. Accordingly, 88 cases had detectable MRD levels ≥10-4, 21 patients had persistent MRD at 10-5, and the remaining 54 cases were MRD-negative. Importantly, MRD-positive patients at 10-5 had similar outcome as compared to cases with MRD levels ≥10-4 (both had median TTP of 31 months; 3-year OS rates were 80% and 74%, respectively) and significantly inferior to that of MRD-negative patients [median TTP not reached (P <.001); 3-year OS rate of 93% (P =.05)].
Conclusions: We showed that the transition from a first-generation 4-color into a second-generation 8-color MFC assay that measured ten-times more cells resulted in increased specificity and sensitivity. MRD detection at the 10-5 level is clinically relevant, since it identifies a subset of patients with inferior survival than MRD-negative cases, similar to that of the overall MRD-positive patient population.
Paiva:Millenium: Consultancy; BD Bioscience: Consultancy; Celgene: Consultancy; Janssen: Consultancy; EngMab AG: Research Funding; Binding Site: Consultancy; Onyx: Consultancy; Sanofi: Consultancy. Puig:Janssen: Consultancy; The Binding Site: Consultancy. Gironella:Celgene Corporation: Consultancy, Honoraria. van Dongen:BD Biosciences (cont'd): Other: Laboratory Services in the field of technical validation of EuroFlow-OneFlow antibody tubes in dried format. The Laboratory Services are provided by the Laboratory of Medical Immunology, Dept. of Immunology, Erasmus MC, Rotterdam, NL; Cytognos: Patents & Royalties: Licensing of IP on Infinicyt software, Patents on EuroFlow-based flowcytometric Diagnosis and Classification of hematological malignancies, Patents on MRD diagnostics, and Patents on PID diagnostics.; Cytognos (continued): Patents & Royalties: Royalty income for EuroFlow Consortium. The Infinicyt software is provided to all EuroFlow members free-of-charge.Licensing of Patent on detection of IgE+ B-cells in allergic diseases. Royalties for Dept. of Immunology, Erasmus MC, Rotterdam, NL; DAKO: Patents & Royalties: Licensing of IP and Patent on Split-Signal FISH. Royalties for Dept. of Immunology, Erasmus MC, Rotterdam, NL; InVivoScribe: Patents & Royalties: Licensing of IP and Patent on BIOMED-2-based methods for PCR-based Clonality Diagnostics.. Royalty income for EuroClonality-BIOMED-2 Consortium ; Immunostep: Patents & Royalties: Licensing of IP and Patents on immunobead-based dection of fusion proteins in acute leukemias and other tumors. Royalties for Dept. of Immunology, Erasmus MC and for EuroFlow Consortium ; BD Biosciences: Other: Educational Services: Educational Lectures and Educational Workshops (+ related travelling costs). The lectures and workshops fully focus on the scientific achievements of the EuroFlow Consortium (No advertisement of products of BD Biosciences). , Patents & Royalties: Licensing of IP and Patent on EuroFlow-based flowcytometric Diagnosis and Classification of hematological malignancies; Royalty income for EuroFlow Consortium.; Roche: Consultancy, Other: Laboratory Services in the field of MRD diagnostics, provided by the Laboratory of Medical Immunology, Dept. of Immunology, Erasmus MC, Rotterdam, NL.. Mateos:Takeda: Consultancy; Onyx: Consultancy; Celgene: Consultancy, Honoraria; Janssen-Cilag: Consultancy, Honoraria. San Miguel:Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Millennium: Membership on an entity's Board of Directors or advisory committees; Onyx: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal