Abstract
Background: Serum and urine immunofixation electrophoreses (SIFE/UIFE) are routinely used for detection of clonal immunoglobulins (Ig) in AL amyloidosis. Serum free light chain (FLC) assays (Freelite®, The Binding Site Ltd., Birmingham, UK) have significantly improved the management of patients with AL amyloidosis by providing quantitative measure for the detection and monitoring of clonal plasma cell disease. However, up to 20% of patients with AL amyloidosis may have uninformative serum free light chain values.
Objective: To assess the quantitative potential of serum Heavy/Light Chain (HLC) pairs (Hevylite®, The Binding Site Ltd., Birmingham, UK) assay in identification of clonal plasma cell disease in AL amyloidosis.
Methods: One hundred and ninety-nine untreated patients with AL amyloidosis were included in this study. Patients with multiple myeloma or B cell lymphoproliferative diseases associated AL amyloidosis were excluded. Serum sampleswere obtained at initial evaluation and stored at -20°C. SIFE/UIFE were performed at the time of sample collection. HLC pairs were assessed by the Hevylite® assay. HLC κ/λ normal ratios (HLCR) were: 1.12-3.21 for IgG κ/λ; 0.78-1.94 for IgA κ/λ; and 1.18-2.74 for IgM κ/λ. FLCs were assessed by the Freelite® assay; FLC κ/λ normal ratio (FLCR) was 0.26-1.65. In 103 cases, FLC testing was performed at the time of sample collection; 96 cases were tested at The Binding Site. Vital status of patients was obtained from either medical records or Social Security Death Index. Follow-up ended in June 2014.
Results: An abnormal HLCR was found in 74 (37.2%), an abnormal FLCR in 163 (81.9%), and SIFE/UIFE positivity in 187 (94%) of 199 patients with AL amyloidosis. Of 36 patients with a normal FLCR, 23 (63.9%) were noted with an abnormal HLCR compared to 51 (31.3%) patients in an abnormal FLCR group (P = 0.001). In total 186/199 (93.5%) patients with AL amyloidosis had abnormalities in either HLCR or FLCR, compared to 187/199 (94%) of patients who were SIFE/UIFE+ (Table 1). The combined use of both FLCR and HLCR yielded quantifiable information in 93.5% of cases; the use of both tests in combination with SIFE/UIFE identified plasma cell clonality in 100% of patients. Seventy-two cases presented with an abnormal HLCR for a single isotype and 2 in multiple Ig isotypes. In all cases, involved LC type of abnormal HLCR matched LC type identified by SIFE/UIFE. None of 12 cases that were negative on the SIFE/UIFE presented with an abnormal HLCR, however, all showed abnormalities in FLCR.
Comparative efficiency of FLCR, HLCR and Serum/Urine Immunofixation in AL Amyloidosis patients.
| . | SIFE/UIFE+ (n=187) . | SIFE/UIFE- (n=12) . | . |
|---|---|---|---|
| HLCR+/FLCR+ | 51 (27.2%) | - | |
| HLCR+/FLCR- | 23 (12.3%) | - | |
| HLCR-/FLCR+ | 100 (53.5%) | 12 (100%) | |
| HLCR-/FLCR- | 13 (7%) | - |
| . | SIFE/UIFE+ (n=187) . | SIFE/UIFE- (n=12) . | . |
|---|---|---|---|
| HLCR+/FLCR+ | 51 (27.2%) | - | |
| HLCR+/FLCR- | 23 (12.3%) | - | |
| HLCR-/FLCR+ | 100 (53.5%) | 12 (100%) | |
| HLCR-/FLCR- | 13 (7%) | - |
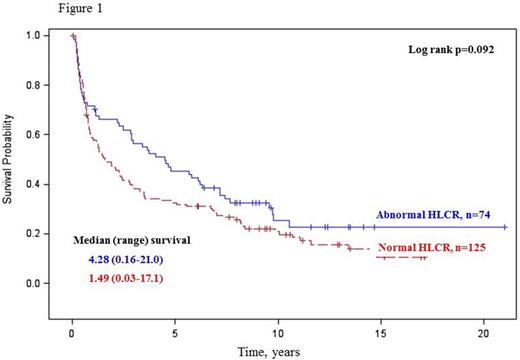
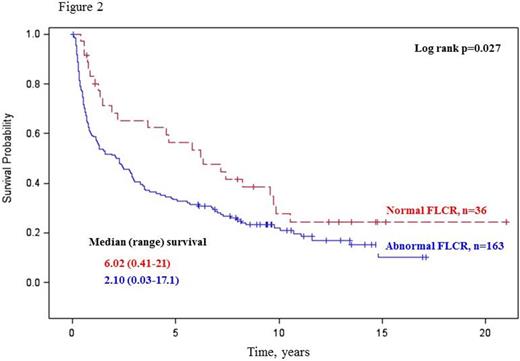
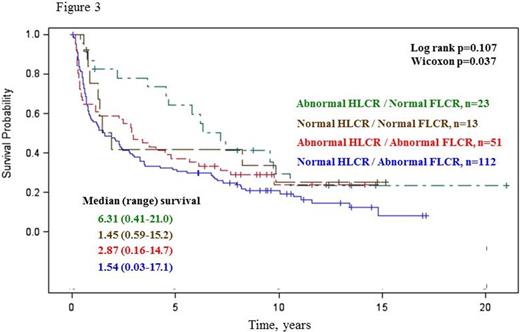
Overall survival was similar in patients with and without abnormal HLCR (Log rank p=0.092; Figure 1), whereas patients with an abnormal FLCR had a significantly inferior overall survival compared to those with a normal FLCR (Log rank p=0.027; Figure 2). Combined use of both HLCR and FLCR demonstrated a trend toward superior overall survival in a group of patients with an abnormal HLCR / normal FLCR (Wilcoxon p=0.037; Log rank p=0.107; Figure 3).
Conclusions: The Hevylite® assay provided information in addition to other laboratory tests for clonal plasma cell disease in AL amyloidosis. The combined use of the HLCR and FLCR provided quantifiable information in 93.5% of patients. The use of both assays in combination with SIFE/UIFE detected clonal disease in all patients. HLCR has potential to quantify clonal disease in patients with uninformative FLCR results. An abnormal HLCR was not predictive of overall survival, while an abnormal FLCR was, in this series of patients. Combined use of HLCR and FLCR could be beneficial in prognostication of outcome in AL amyloidosis.
McConnell:The Binding SIte: Employment. O'hara:The Binding Site: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal