Abstract
The clinical success of PI3K and Btk inhibitors that block upstream BCR signals, has been primarily attributed to inhibition of adhesion and migration of CLL cells. However, the effects of these inhibitors on the proliferative capacity of CLL cells is largely unknown. Interestingly, in vitro BCR triggering does not induce proliferation of CLL cells. Instead, we have found that activated T cell-derived signals, specifically CD40L and IL-21, initiate CLL proliferation in vitro and can be traced in CLL lymph node samples from patients [Pascutti et al., Blood 2013]. Targeting the underlying signaling pathways may be clinically relevant, and therefore we aimed to characterize the molecular mechanisms underlying antigen-independent proliferation.
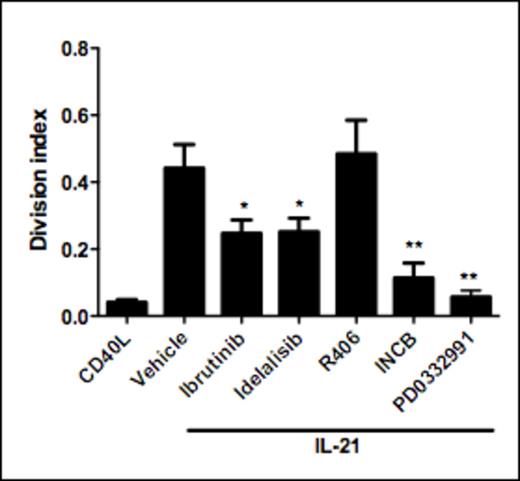
In the present study we applied CD40L/IL-21 stimulation to induce proliferation, and tested various specific kinase inhibitors for blocking potential. Surprisingly, the Btk inhibitor ibrutinib significantly suppressed CD40L/IL-21-induced proliferation (Figure 1). Similarly, inhibition of PI3Kδ with idelalisib dampened proliferation. While neither ibrutinib nor idelalisib affected CD40L-induced NF-kB or IL-21 induced STAT3 phosphorylation, both compounds efficiently blocked downstream ERK1/2 phosphorylation. Impact of idelalisib and ibrutinib was non-synergistic, suggesting that PI3K and Btk are acting in concert. In contrast with the inhibitory effects of the two compounds above, no effect of CD40L/IL-21 induced proliferation was observed following inhibition of SYK activity with R406 (tamatinib). This strongly suggests a role for PI3K and Btk signaling independent of BCR-signaling.
We demonstrate that antigen-independent proliferation completely depends on downstream CDK4-activity, which can be blocked by the CDK4-inhibitor PD0332991.
IL-21 signaling is absolutely required for antigen-independent proliferation as treatment with the pan-JAK inhibitor INCB abrogates proliferation. Moreover, immunohistochemical staining of lymph nodes from CLL patients indicated the presence of phospho-STAT3 in CLL cells at lymph node sites, suggesting a contribution of JAK/STAT signaling to in vivo proliferation.
In conclusion, our data strongly suggest that non-BCR signals contribute to proliferation of CLL cells. Since the most recent clinical data point to development of resistance against ibrutinib, targeting non-BCR pathways may offer additional venues for treatment.
CD40L/IL-21 induced proliferation can be inhibited with kinase inhibitors that target kinases involved in BCR-signaling
CD40L/IL-21 induced proliferation can be inhibited with kinase inhibitors that target kinases involved in BCR-signaling
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal