Abstract
INTRODUCTION
Anemia is the most frequent cytopenia in lower-risk MDS. Erythropoietic-stimulating agents (ESAs) are commonly used in these patients. The use of ÒclassicalÓ parameters (EPO and ferritin levels) and the revised IPSS (IPSS-R) has been proposed1 (SantiniÕs score) to predict response to ESAs and overall survival (OS) among patients with lower risk MDS by IPSS and a favorable Nordic group score2.
OBJECTIVES
The main objective of the study was to evaluate overall response rate (ORR) to ESAs and OS according to the proposed SantiniÕs score in an independent and large cohort of anemic lower risk MDS patients receiving treatment with ESAs.
METHODS
Data from 530 anemic patients with low/int1 risk IPSS de novo MDS (according to FAB and WHO criteria) and sufficient follow-up data available were recorded in Spresas3 (SPanish Registry of Erythropoietic Stimulating Agents Study from GESMD). Two hundred and twenty six patients (42.6% of the patients) were selected according to specific criteria regarding the published SantiniÕs score1: Hb level </=10 g/dL, serum erythropoietin (EPO) <500 mU/mL and ESA (EPO alfa or B 40000-60000IU/week, or darbepoetin 150-300 ug/week). Applying 1 point to each of the following unfavorable variables for response to ESA, EPO>200mU/mL(=1), serum ferritin (SF) >350 ng/mL(=1) and IPSS-R very low=0, low=1, intermediate=2 and high=3) yielded a score ranging from 0 to 5. ESAs response rate and overall survival were analysed according to these score. Response to treatment was evaluated according to IWG 2006 response criteria and a multivariate logistic regression analysis was used to identify independent predictors of erythroid response (ER). OS were defined as the time between diagnosis and the corresponding event or last follow up (Feb 2015) and were analyzed using univariable and multivariable Cox proportional hazards regression methods.
RESULTS
Median age was 77 years (interquartile range [IQR] 25%-75%: 71-83 y), median Hb level at start of treatment was 10 g/dL (IQR25-75: 9-10), median EPO level was 90 (IQR25-75: 27,25-108) and median ferritin level was 338,5 (IQR25-75: 146,5-568,75). Among 139 patients with this data available, 85 patients (61,1%) were RBC transfusion dependent before ESAs treatment. Median time from diagnosis to ESAs treatment was 82 (IQR25-75: 27-353) days. According to the IPSS, 68.6% (N=155) and 31.4% (N=71) were in low and Int-1 risk groups, respectively. Regarding IPSS-R, 23% (N=52), 66.8% (N=151), 9.7% (N=22) and 0.4% (N=1) were in very low, low, intermediate and high risk, respectively. ORR to ESA treatment was 71.2% (N=161), with a median duration of response of 2.06 years. Prognosis factors of ER showed a trend toward to a higher ER among patients in the lower IPSS-R (P>0.05), low IPSS (p=0.039) and lower EPO levels (p<0.0001) while in multivariate analysis EPO level was confirmed as most significant variable associated to ER (p<0.001).
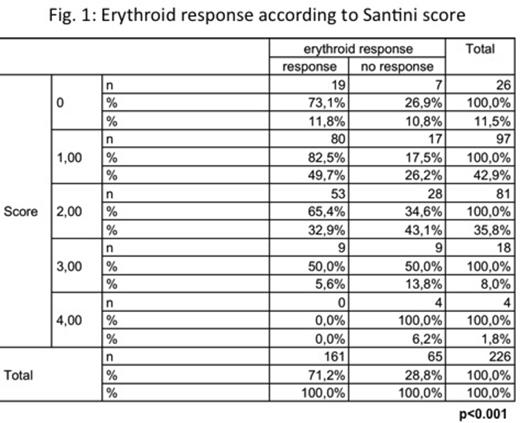
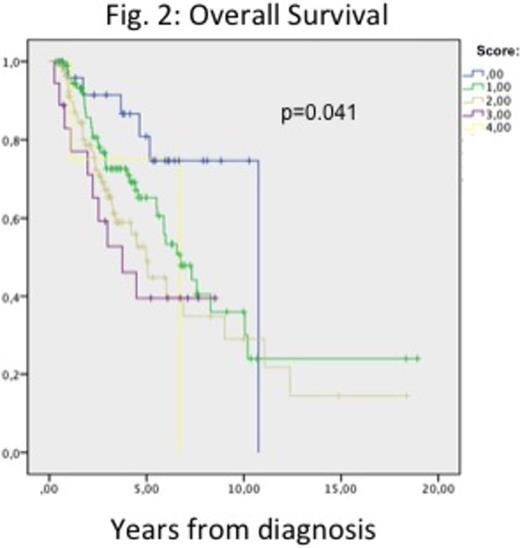
According to SantiniÕs score, 11.5%(N=26), 42.9%(N=97), 25.8%(N=81), 8%(N=18) and 1.8%(N=4) of the patients were in the 0, 1, 2 3 and 4 score. Erythroid response was better for patients in the lower scores, with response rates of 73.1%, 82.5%, 65.4%, 50% and 0%, for patients in 0, 1, 2, 3 and 4 score, respectively (p<0.001, figure 1). After a median follow up of 3.1 years, median OS from diagnosis was 4.99 years. Interestingly, median OS from diagnosis was clearly related to SantiniÕs score (10.7 years, 6.7y, 4.9y, 3.7y and 6.7y for patients with 0, 1, 2, 3, and 4 points, respectively, p=0.041, Figure 2) whereas median OS from start of ESAs showed also some trend (p=0.26).
CONCLUSIONS
The present study confirms that SantiniÕs score is useful to identify patients with a higher probability of response to ESAs and better OS among lower risk MDS patients with an expected favorable response to ESA according to Nordic group score.
Spresas study was partly supported by Janssen
1.-Santini et al, Blood 122(13), 2013.
2.-Hellstršm-Lindberg, Br J Haematol 120(6), 2003.
3.-D'ez Campelo, EHA 2015 meeting, P244.
Díez Campelo:Novartis: Research Funding, Speakers Bureau; Janssen: Research Funding; Celgene: Research Funding, Speakers Bureau. Off Label Use: Use of erythropoietic stimulating agents for anemia in patients with myelodysplastic syndromes. Ramos:JANSSEN: Honoraria, Membership on an entity's Board of Directors or advisory committees; AMGEN: Consultancy, Honoraria; NOVARTIS: Consultancy, Honoraria; CELGENE: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Falantes:Celgene: Honoraria. Garcia:Celgene: Research Funding. Sanz:JANSSEN CILAG: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal