Abstract
Background
The standard first-line therapy for higher-risk myelodysplastic syndromes (MDS) are the hypomethylating agents (HMA). While clinical characteristics, molecular markers, and karyotype can contribute to predicting prognosis in MDS, these parameters have not identified differential response rates between the HMAs decitabine (DAC) and azacitidine (AZA). Gender differences have been associated with varied outcomes in multiple cancers, and are thought to be related to differences in disease biology, treatment response, or adherence to therapy. Relevant to MDS, gender can effect expression of cytidine deaminase, which inactivates the HMAs. Male have increased levels compared to females. The impact of gender on response to HMAs is not established.
Methods
A dataset from the MDS CRC database was analyzed. Patients (pts) were diagnosed with higher-risk MDS (International Prognostic Scoring System (IPSS) Int-2 or High) and per 2008 WHO criteria and were treated with AZA or DAC as first-line therapy. Response was assessed per International Working Group (IWG) criteria (2006). The IPSS and its revision (IPSS-R) were calculated at presentation. Missing data were multiply imputed 100 times using the mice approach. Differences among variables were evaluated by the chi-square test and Mann-Whitney U test for categorical and continuous variables, respectively, with continuous variables summarized by median and range. Overall survival (OS) was calculated from presentation date to date of death or last follow up. Propensity scores were calculated for DAC vs AZA using all variables available pre-treatment. Propensity weighted Cox proportional analysis and log-rank tests were used to model and test OS.
Results
Of 625 higher-risk MDS pts, 33.7% were women (Table 1). The median OS for the cohort was 16.9 months (95% CI 15.6-18.2); 17.8 months for men (95% CI 16.4-19.2) and 15.0 months for women (95% CI 12.1-18.6). Approximately one third of pts received DAC as front line HMA in both gender groups. While most variables had similar distributions between DAC and AZA within genders, two variables showed differences between HMA treatments in both genders: bone marrow blast percentage at diagnosis and IPSS-R cytogenetic category, with blast percentage consistently higher in the DAC treated set. In addition, DAC-treated females were on treatment longer than AZA-treated females
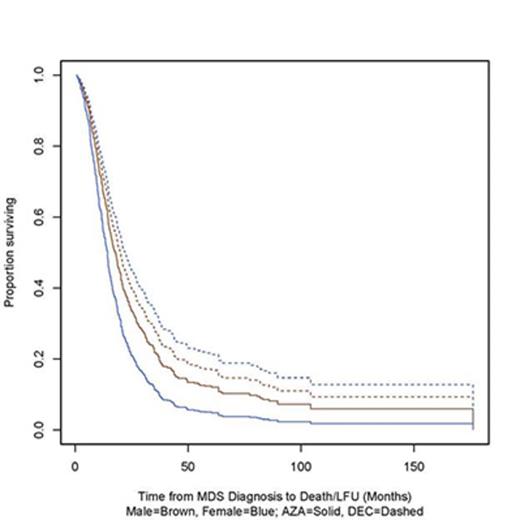
There was no difference in median OS across gender (p=0.33). DAC-treated pts had marginally better OS than AZA pts (p=0.043), (median OS of 18.7 months vs 16.3 months), but the difference varied strongly by gender (Figure 1). Female DAC pts had much better OS than female AZA pts (p=0.0014), with a median OS of 21.2 months (95% CI 16.1-27.9) versus 13.1 months (95% CI 10.7-15.9). Males showed no significant difference (p=0.59), with a median OS of 18.3 months (95% CI 14.9-22.2) compared to 17.6 years (95% CI 16.0-19.5). Female DAC survival improvement remained significant in a Cox PH analysis after adjusting for cytogenetic category and bone marrow blast % at diagnosis. (Figure 1)
Conclusion
Women with higher-risk MDS may live longer when treated with DAC than with AZA. While factoring in gender as a variable in therapeutic choice between the HMAs remains premature, future prospective investigations of both drugs in men and women are warranted.
Patient characteristics by Sex
| . | Female N=210, 33.7% . | Male N=415, 66.3% . | ||||
|---|---|---|---|---|---|---|
| Parameter | DAC | AZA | P-value | DAC | AZA | P-value |
| N=72 34.3% | N=138 65.7% | N=130 31.3% | N=285 68.7% | |||
| Age, years | 67.4 35-84 | 68.9 36-91 | 0.222 | 70.0 38-89 | 70.8 31-99 | 0.641 |
| ANC X 109 L | 1.06 0.04-27.7 | 0.83 0.10-20.4 | 0.085 | 1.04 0.02-44.9 | 1.01 0.01-42.6 | 0.900 |
| Platelets X 109 L | 57.8 2.0-412.4 | 59.4 4.9-655.2 | 0.820 | 55.6 3.0-654 | 66.2 2.5-451.3 | 0.095 |
| Hemoglobin g/dL | 9.53 4.38-12.90 | 9.27 4.91-14.04 | 0.271 | 9.40 4.10-13.93 | 9.13 3.00-15.91 | 0.086 |
| Bone marrow blasts % | 13.0 0.27-29 | 10.0 0-84 | <0.001 | 13.1 0-29.1 | 9.9 0-83 | <0.001 |
| Time on HMA (months) | 6.0 0.16-30.1 | 4.2 0.14-41.3 | 0.055 | 4.7 0.10-38.8 | 5.4 0.03-55.0 | 0.840 |
| IPSS-R Category Low/Very Low Intermediate High Very High | 0.3 12.5 36.9 50.4 | 0.1 10.2 29.5 60.2 | 0.496 | 0.1 11.8 33.6 54.5 | 1.8 9.5 35.3 53.3 | 0.567 |
| IPSS-R Cytogenetic Cat Very Poor Poor Intermediate Good/Very Good | 47.3 3.2 21.1 29.7 | 43.1 21.6 16.9 18.8 | 0.0011 | 42.4 8.1 18.5 31.0 | 42.4 11.7 25.3 20.6 | 0.086 |
| Progression to AML (Y) | 46.5 | 47.4 | 0.936 | 44.8 | 43.1 | 0.803 |
| . | Female N=210, 33.7% . | Male N=415, 66.3% . | ||||
|---|---|---|---|---|---|---|
| Parameter | DAC | AZA | P-value | DAC | AZA | P-value |
| N=72 34.3% | N=138 65.7% | N=130 31.3% | N=285 68.7% | |||
| Age, years | 67.4 35-84 | 68.9 36-91 | 0.222 | 70.0 38-89 | 70.8 31-99 | 0.641 |
| ANC X 109 L | 1.06 0.04-27.7 | 0.83 0.10-20.4 | 0.085 | 1.04 0.02-44.9 | 1.01 0.01-42.6 | 0.900 |
| Platelets X 109 L | 57.8 2.0-412.4 | 59.4 4.9-655.2 | 0.820 | 55.6 3.0-654 | 66.2 2.5-451.3 | 0.095 |
| Hemoglobin g/dL | 9.53 4.38-12.90 | 9.27 4.91-14.04 | 0.271 | 9.40 4.10-13.93 | 9.13 3.00-15.91 | 0.086 |
| Bone marrow blasts % | 13.0 0.27-29 | 10.0 0-84 | <0.001 | 13.1 0-29.1 | 9.9 0-83 | <0.001 |
| Time on HMA (months) | 6.0 0.16-30.1 | 4.2 0.14-41.3 | 0.055 | 4.7 0.10-38.8 | 5.4 0.03-55.0 | 0.840 |
| IPSS-R Category Low/Very Low Intermediate High Very High | 0.3 12.5 36.9 50.4 | 0.1 10.2 29.5 60.2 | 0.496 | 0.1 11.8 33.6 54.5 | 1.8 9.5 35.3 53.3 | 0.567 |
| IPSS-R Cytogenetic Cat Very Poor Poor Intermediate Good/Very Good | 47.3 3.2 21.1 29.7 | 43.1 21.6 16.9 18.8 | 0.0011 | 42.4 8.1 18.5 31.0 | 42.4 11.7 25.3 20.6 | 0.086 |
| Progression to AML (Y) | 46.5 | 47.4 | 0.936 | 44.8 | 43.1 | 0.803 |
Overall survival by HMA and Sex (and breakdown by HMA) from Time of Presentation to CRC Institution
Overall survival by HMA and Sex (and breakdown by HMA) from Time of Presentation to CRC Institution
Komrokji:Incyte: Consultancy; Novartis: Research Funding, Speakers Bureau; Pharmacylics: Speakers Bureau; Celgene: Consultancy, Research Funding. Steensma:Celgene: Consultancy; Amgen: Consultancy; Incyte: Consultancy; Onconova: Consultancy. Sekeres:Celgene Corporation: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal