Abstract
Background: Sickle cell disease (SCD) is one of the world's most common inherited diseases, due to a point mutation in hemoglobin that leads to the polymerization of hemoglobin S, giving red blood cells (RBCs) their sickle shape. The hallmarks of SCD are hemolysis and vaso-occlusion. Vaso-occlusive crises (VOC) frequently occur in response to inflammatory and hypoxic conditions such as infections, surgery, etc. Pain is a major defining characteristic of SCD starting early in life and continuing throughout adulthood. VOC-induced pain can be chronic or episodic and unpredictable, requiring frequent hospitalizations and analgesics. Opioids, predominantly morphine, have been the mainstay treatment for pain management of SCD but pose a serious challenge attributed to the many adverse side effects, including tolerance, respiratory depression, sedation, nausea, constipation, pruritus, and dependence. Given that analgesics characteristically used to treat pain appear to be relatively ineffective in alleviating pain associated with SCD as well as display their own dose-limiting adverse effects, there exists a requisite need for superior pharmacological agents in its treatment. Here we reveal that the bivalent ligand MCC22, is highly effective as an analgesic in a murine model of SCD. MCC22 (MW=1255) contains both mu agonist and chemokine CCR5 antagonist pharmacophores that are linked through a 22-atom-spacer. It was designed specifically to target the mu opiate receptor (MOR)-CCR5 heteromer, as there is evidence for crosstalk between MOR and CCR5 in cultured cells that reduces the efficacy of opioid analgesics employed in SCD. Intrathecal (i.t.) MCC22 was potent in reducing the mechanical and heat hyperalgesia in Complete Freund's Adjuvant (CFA) and lipopolysaccharide (LPS) inflammatory pain assays. ED50s for the i.t. administration were 0.019 and 0.015 pmol/mouse, respectively. MCC22 when given intraperitoneally (i.p.) to LPS pretreated mice had an ED50 of 5.6 µmol/kg. We next investigated whether MCC22 would be effective in alleviating pain in SCD mice.
Methods:Townes transgenic humanized mice expressing sickle hemoglobin (HbSS / HbAS) and normal human hemoglobin (HbAA) controls (4-12 weeks of age, 20-25g) were used in this study. Mice were characterized for hyperalgesia by quantifying cutaneous mechanical sensitivity of the hind paw and forelimb grip force. Mechanical sensitivity of the hind paw was evaluated by determining the frequency of withdrawal responses and paw withdrawal threshold. The frequency of paw withdrawal evoked by a standard von Frey monofilament with a bending force of 9.3 mN applied to the plantar surface of the hind paws was determined from 10 trials on each paw. Withdrawal threshold was determined using an electronic von Frey anesthesiometer pressed against the plantar surface with increasing force until withdrawal occurred. To assess deep tissue hyperalgesia, the tensile force of peak forelimb exertion was measured using a computerized grip force meter. To evaluate the analgesic effects of MCC22, paw withdrawal frequency was determined before and at various times after administration of 8.0 µmol/kg i.p.
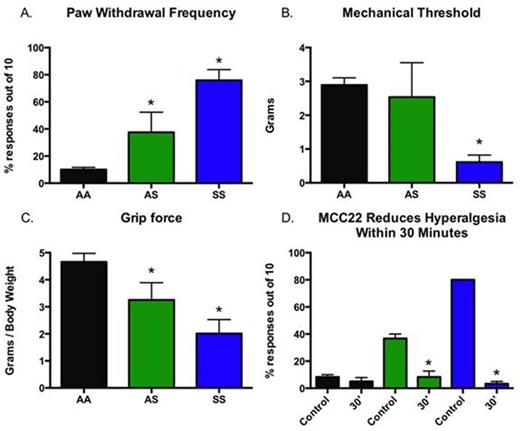
Results: Pain Characterization: HbSS sickle mice exhibited robust mechanical hyperalgesia as shown by a significant increase in paw withdrawal frequency compared to HbAS and HbAA controls (Figure1A, p<0.001). Paw withdrawal frequency in HbAS mice was also greater than those for HbAA mice (p<0.001), but less than HbSS mice. HbSS mice had lower mechanical withdrawal thresholds compared to both HbAS and HbAA mice (Figure 1B, p<0.001), while HbAS did not differ from HbAA mice. HbSS and HbAS mice also displayed lower forelimb grip force compared to HbAA mice (Figure 1C, p<0.001), although grip force in HbSS was lower than HbAS mice (p<0.001). Administration of MCC22 (8.0 µmol/kg i.p.) reduced hyperalgesia within 30 minutes as evidenced by a decrease in paw withdrawal frequency in both HbSS and HbAS mice (Figure 1D, p<0.001).
Conclusions: Hyperalgesia was demonstrated in both HbAS and HbSS mice. MCC22 potently attenuated mechanical hyperalgesia in these SCD mice. The use of bivalent ligands that target heteromers involved in signaling pain may offer novel and effective treatments for pain in patients with SCD. We speculate that an analgesic with potential anti-inflammatory and mu-agonist activity may provide a novel approach to sickle pain.
Belcher:CSL Behring: Research Funding; Seattle Genetics: Research Funding; Biogen Idec: Research Funding. Vercellotti:Biogen Idec: Research Funding; CSL Behring: Research Funding; Cydan: Research Funding; Seattle Genetics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal