Abstract
Introduction
The additional benefit of consolidation radiotherapy (RT) following chemotherapy in limited stage DLBCL remains controversial. Previous 5 randomized trials, 4 trials before rituximab era (SWOG 8736, ECOG 1484, GELA 93-1 and 93-4 studies) and 1 trial with R-CHOP combination (Lysa/Goelams 02-03), could not demonstrate a remarkable benefit of RT. The purpose of this study is to explore the benefit of combined modality treatment including the combination of RT and rituximab (R)-based chemotherapy in limited stage DLBCL.
Methods
From the 4,371 patients in a multi-institutional registry of newly diagnosed lymphoma in Thailand between 2007-2014, there were a total of 2,399 patients with DLBCL. We included patients with limited stage DLBCL receiving CHOP/CHOP-liked chemotherapy +/- R and +/- RT. The baseline patient characteristics and clinical outcomes were analyzed according to treatment modalities.
Results
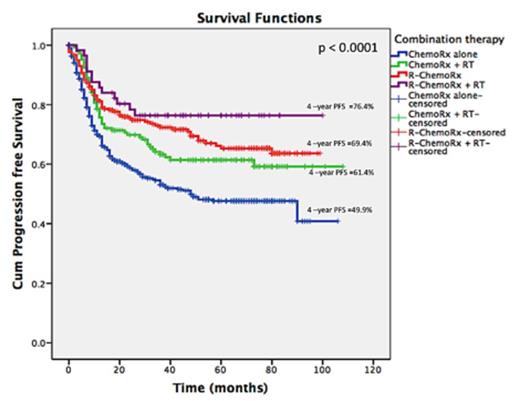
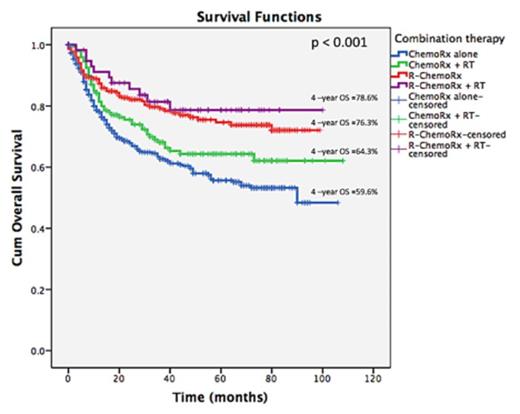
A total of 816 patients with a median age of 56 years (range, 15-91) were included in the study. Male:female was 1:1.1. Majority of patients had primary extranodal diseases (58%), stage II (68%), good performance status (89%), normal LDH (55%) and no B-symptoms (59%). The IPI scores were 0 (30.6%), 1(45%), 2 (20%) and 3 (3.9%), respectively. The modalities of treatment were CHOP alone (48.5%), R-CHOP (26.5%), CHOP+RT (17.9%) and R-CHOP+RT (7.1%). Patients in R-CHOP group were older than in other groups (P=0.001) (Table 1).There was a higher proportion of patients in stage II disease in R-CHOP (74.1%) and R-CHOP+RT (74.1%) groups than in CHOP (66.9%) and CHOP+RT (57.5%) groups (P=0.007), whereas the patients were relatively equally distributed between the IPI scores. Complete response (CR) rate was higher in R-CHOP+RT group (82.8%) than in R-CHOP (75%), CHOP+RT (70%) and CHOP groups (58%) (P<0.001). CR was independently associated with consolidation RT (OR 0.65, 95%CI: 0.45-0.97), rituximab-based therapy (OR 0.66; 95%CI: 0.55-0.79) and IPI score (OR 1.90; 95%CI: 1.41-2.57). With a median follow up of 52 months, 4-year progression free survival (PFS) were 76.4%, 69.4%, 61.4% and 49.9% in R-CHOP+RT, R-CHOP, CHOP+RT and CHOP alone, respectively, (P<0.001) (Figure. 1). The corresponding figures of 4-year OS for each group were 78.6%, 76.3%, 64.3% and 59.6%, respectively (P<0.001) (Figure. 2). Multivariate analysis showed that factors associated with survivals were consolidation RT (HR for PFS 0.63, 95%CI: 0.48-0.82; HR for OS 0.70, 95%CI: 0.53-0.94), rituximab-based therapy (HR for PFS 0.72, 95%CI: 0.64-0.83; HR for OS 0.69, 95%CI: 0.60-0.81) and IPI scores (HR for PFS 1.87, 95%CI: 1.54-2.26; HR for OS 1.96, 95%CI: 1.60-2.41)
Conclusion
In limited stage DLBCL, consolidation RT plus R-CHOP yielded a superior outcome compared to R-CHOP, CHOP + RT and CHOP alone. Thus, RT continues to have an important role and should not be omitted in management of limited stage DLBCL in rituximab era.
Comparison of characteristics of patients according to treatment modalities
| Clinical characteristics . | CHOP alone (n= 396) . | CHOP + RT (n = 146) . | R-CHOP alone (n = 216) . | R-CHOP + RT (n = 58) . | p-value . |
|---|---|---|---|---|---|
| Male | 211 (53%) | 75(51.4%) | 125 (57.9%) | 24 (41.4%) | 0.14 |
| Median age (years) | 56 (15-90) | 52 (15-86) | 60 (17-91) | 55 (15-81) | 0.001 |
| Age ≥ 60 | 155 (39.1%) | 51 (34.9%) | 109 (50.5%) | 20 (34.5%) | 0.008 |
| Primary extranodal disease | 226 (57.1% | 79 (54.1%) | 136 (63.0% | 34 (58.6%) | 0.35 |
| Stage Stage I Stage II | 131 (33.1%) 269 (66.9%) | 62 (42.5%) 84 (57.5%) | 56 (25.9%) 160 (74.1) | 15 (25.9%) 43 (74.1%) | 0.007 |
| B-symptom | 116 (32.6%) | 47 (36.2%) | 58 (31.4%) | 13 (28.9%) | 0.76 |
| HIV seropositivity | 15 (4.6%) | 9 (7.0%) | - | - | 0.004 |
| High serum LDH | 173 (43.7%) | 74 (50.7%) | 95 (44.0%) | 26 (44.8%) | 0.51 |
| ECOG ≥ 2 | 31 (7.8%) | 22 (15.1%) | 25 (11.6%) | 7 (12.1%) | 0.08 |
| IPI Low (IPI =0-1) Low-intermediate (IPI =2) High-intermediate (IPI = 3) | 312 (78.8%) 75 (18.9%) 9 (2.3%) | 107(73.3%) 32 (29.1%) 7 (4.8%) | 157 (72.7%) 48 (22.2%) 11 (5.1%) | 46 (79.3%) 9 (15.5%) 3 (5.2%) | 0.35 |
| Response Overall response Complete response | 258 (65.2%) 230 (58.1%) | 116(79.4%) 103(70.5%) | 179 (82.9%) 162 (75%) | 51 (88%) 48 (82.8%) | <.0001 |
| Clinical characteristics . | CHOP alone (n= 396) . | CHOP + RT (n = 146) . | R-CHOP alone (n = 216) . | R-CHOP + RT (n = 58) . | p-value . |
|---|---|---|---|---|---|
| Male | 211 (53%) | 75(51.4%) | 125 (57.9%) | 24 (41.4%) | 0.14 |
| Median age (years) | 56 (15-90) | 52 (15-86) | 60 (17-91) | 55 (15-81) | 0.001 |
| Age ≥ 60 | 155 (39.1%) | 51 (34.9%) | 109 (50.5%) | 20 (34.5%) | 0.008 |
| Primary extranodal disease | 226 (57.1% | 79 (54.1%) | 136 (63.0% | 34 (58.6%) | 0.35 |
| Stage Stage I Stage II | 131 (33.1%) 269 (66.9%) | 62 (42.5%) 84 (57.5%) | 56 (25.9%) 160 (74.1) | 15 (25.9%) 43 (74.1%) | 0.007 |
| B-symptom | 116 (32.6%) | 47 (36.2%) | 58 (31.4%) | 13 (28.9%) | 0.76 |
| HIV seropositivity | 15 (4.6%) | 9 (7.0%) | - | - | 0.004 |
| High serum LDH | 173 (43.7%) | 74 (50.7%) | 95 (44.0%) | 26 (44.8%) | 0.51 |
| ECOG ≥ 2 | 31 (7.8%) | 22 (15.1%) | 25 (11.6%) | 7 (12.1%) | 0.08 |
| IPI Low (IPI =0-1) Low-intermediate (IPI =2) High-intermediate (IPI = 3) | 312 (78.8%) 75 (18.9%) 9 (2.3%) | 107(73.3%) 32 (29.1%) 7 (4.8%) | 157 (72.7%) 48 (22.2%) 11 (5.1%) | 46 (79.3%) 9 (15.5%) 3 (5.2%) | 0.35 |
| Response Overall response Complete response | 258 (65.2%) 230 (58.1%) | 116(79.4%) 103(70.5%) | 179 (82.9%) 162 (75%) | 51 (88%) 48 (82.8%) | <.0001 |
Khuhapinant:Roche: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal