Abstract
Background: Lenalidomide with dexamethasone (Rd) is a standard of care for patients with previously untreated multiple myeloma. SWOG S0777, a randomized phase III trial, has compared Rd with bortezomib, lenalidomide and dexamethasone (VRd). The primary end point is progression-free survival (PFS) using a pre-specified one-sided stratified log rank test at a significance level of 0.02. The stratification factors are International Staging System (ISS) stage (I, II or III) and intent to transplant (yes or no), a total of 6 strata. Overall response rate (ORR), overall survival (OS) and safety are secondary end points.
Methods: This analysis includes 474 patients evaluable for survival endpoints: 232 patients were randomized to Rd and 242 patients to VRd. Rd patients received lenalidomide 25 mg/day on days 1-21 and dexamethasone 40 mg/day on days 1, 8, 15 and 22 of a 28-day cycle. VRd patients received lenalidomide 25 mg/day on days 1-14 and dexamethasone 20/mg/day on days 1, 2, 4, 5, 8, 9, 11 and 12 plus bortezomib 1.3 mg/m2 IV push on days 1, 4, 8 and 11 of a 21-day cycle. All patients received aspirin 325 mg/day and VRd patients received HSV prophylaxis per institutional standard. Induction was six 28-day cycles of Rd and eight 21-day cycles of VRd followed by Rd maintenance for all patients until progression, unacceptable toxicity or withdrawal of consent. Initial analyses utilized the pre-specified one-sided stratified log rank test.
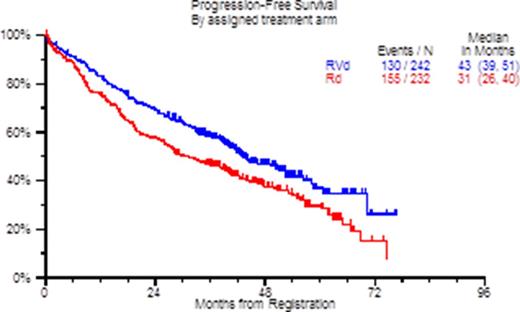
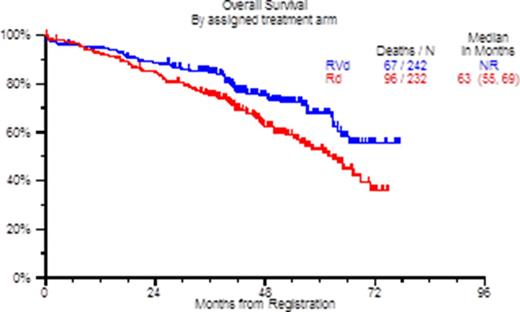
Results: Data are presented for VRd followed by Rd throughout. Between 2008 and 2012, 525 patients from 48 institutions were randomized. Fifty-one patients, 29 randomized to Rd and 22 randomized to RVd, were ineligible for the following reasons: missing, insufficient or early or late baseline labs (40); not meeting requirements of measurable disease (6); inadequate marrow function (1); inadequate creatinine clearance (1); prior malignancy (1); prior therapy (1); and more than 2 weeks of prior steroid therapy (1). The pre-specified significance level of 0.02 was reached in the log rank testing. The stratified hazard ratio (HR) was 0.742 (96% Wald confidence interval: 0.579, 0.951), and the one-sided stratified log rank p-value for PFS (VRd vs. Rd) was 0.0066. The OS was improved for VRd vs. Rd with HR = 0.666; two-sided log-rank p-value = 0.0114. The PFS and OS survival charts are displayed below. Median PFS was 43 months (VRd) versus 31 months (Rd). Median OS was not reached (VRd) versus 63 months (Rd). Patient characteristics were well-matched between VRd and Rd with the exception of fewer women (37% vs. 47%: P = 0.033) and fewer older patients (≥ 65 years 38% vs. 48%: P = 0.042) receiving VRd. With univariate Cox regression analysis correlates of better PFS/OS were: use of VRd (HR 0.72/0.65; P = 0.006); hemogoblin ≥10 g/dl (HR 1.17/1.43; P = 0.2/0.026) and lower ISS disease stage (HR 1.35/1.98; P = 0.014/< 0.001). The ORR for VRd was 71.07% versus 63.79% for Rd. The adverse events by CTC category and toxicity category were fairly well balanced. The most common hematologic adverse events (≥ Grade 3 and at least possibly attributable to therapy) were low hemoglobin (RVd=13%; Rd=16%), leukopenia (RVd=14%; Rd=16%), lymphopenia (RVd=23%; Rd=18%), neutropenia (RVd=19%; Rd=21%), and thrombocytopenia (RVd=18%; Rd=14%). The most common non-hematologic adverse events (≥ Grade 3 and at least possibly attributable to therapy) were: fatigue (RVd=16%; Rd=14%), sensory neuropathy (RVd=23%; Rd=3%), hyperglycemia (RVd=7%; Rd=11%), thrombosis/embolism (RVd=8%; Rd=9%), hypokalemia (RVd=9%; Rd=6%), muscle weakness (RVd=7%; Rd=4%), diarrhea (RVd=8%; Rd=2%), and dehydration (RVd=8%; Rd=2%). As expected ≥ Grade 3 neuropathy was more frequent with VRd (24% vs. 5%: P < 0.0001). Sixteen patients experienced a second primary malignancy, 7 (3%) on VRd and 9 (4%) on Rd.
Conclusion: The addition of bortezomib to lenalidomide dexamethasone for induction therapy in previously untreated myeloma results in a statistically significant and clinically meaningful improvement in PFS as well as better OS. VRd had an acceptable safety and tolerability profile despite increased neurotoxicity and represents a potential new standard of care.
Support: NIH/NCI/NCTN grants CA180888, CA180819, CA180821, CA180820; and in part by Millennium Pharmaceuticals, Inc., The Takeda Oncology Company, for provision of study drug.
Durie:Johnson & Johnson: Consultancy; Takeda: Consultancy; Onyx: Consultancy; Celgene: Consultancy. Abidi:Millennium: Research Funding. Epstein:University of Arkansas for Medical Sciences: Employment. Reu:Takeda/Millennium: Research Funding; Novartis: Research Funding; Celgene: Research Funding. Orlowski:BioTheryX, Inc.: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Research Funding; Spectrum Pharmaceuticals: Research Funding; Onyx Pharmaceuticals: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Millennium Pharmaceuticals: Consultancy, Research Funding; Acetylon: Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; Forma Therapeutics: Consultancy; Array BioPharma: Consultancy, Research Funding. Barlogie:Dana Farber Cancer Institute: Other: Travel Stipend; International Workshop on Waldenström's Macroglobulinemia: Other: Travel Stipend; ComtecMed- World Congress on Controversies in Hematology: Other: Travel Stipend; European School of Haematology- International Conference on Multiple Myeloma: Other: Travel Stipend; Celgene: Consultancy, Research Funding; Millennium: Consultancy, Research Funding; Myeloma Health, LLC: Patents & Royalties: Co-inventor of patents and patent applications related to use of GEP in cancer medicine licensed to Myeloma Health, LLC; Multiple Myeloma Research Foundation: Other: Travel Stipend.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal