Abstract
Background: Given the consistent antitumor activity and favorable toxicity profile in heavily pretreated patients, BDM represents a suitable platform for combination with target-based agents in the setting of recurrent HL. The anti-CD30 antibody-monomethyl auristatin E (MMAE) conjugate brentuximab vedotin (BV) appears a most valuable candidate by coupling an impressive clinical activity with the lack of serious overlapping toxicities with BDM. While the combination of BDM and BV is being evaluated in Phase II studies, no information is available as to possible mechanisms through which BDM may regulate the antitumor efficacy of BV towards HL cells.
Methods: We evaluated the effects of acute and extended exposure to BDM on CD30 expression and sensitivity to the cytotoxic activity BV in the HL cell line L1236. Cells were cultured in the presence of BDM at its IC50 for different time points of acute exposure. Through continuous exposure to increasing concentrations (25.0, 50.0, 75.0, 100 micromol/L) of BDM, we then performed a serial in vitro selection for BDM-resistant (R) L1236 cell clones and determined their CD30 expression by flow cytometry, qRT-PCR, and Western analysis.
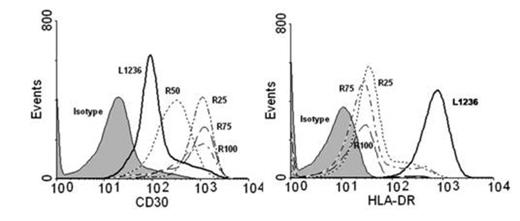
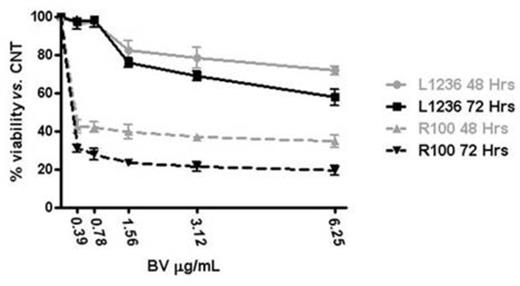
Results: Acute exposure to BDM (48 to 72 hrs) of L1236 cells led to a sizeable upregulation of CD30 as shown by flow cytometry. This effect was unsustained since CD30 intensity returned to baseline levels upon culturing in BDM-free medium for one week. Expression of other surface antigens, i.e. HLA-DR, was unaffected by BDM while acute exposure to doxorubicin and other cytotoxic drugs did not modify the CD30 expression. We established four L1236-derived cell clones (R25, R50, R75 and R100) able to proliferate across the different concentrations of BDM with growth/viability curves superimposable to native cells. Clonal identity among clones and with parental cells was confirmed by sequencing of V3-21 (FR2/FR3) and JH3-JH4 Ig DNA regions. All R-clones displayed an up to 900% increase in CD30 median fluorescence intensity, relative to native L1236 cells (Figure 1A). The sustained CD30 upregulation was confirmed by qRT-PCR and Western blotting since R-clones expressed up to 10-fold higher levels of CD30-specific mRNA and CD30-specific 120 kDa components as compared to parental cells. To rule out a general upregulation of surface molecules as a result of the chronic exposure to BDM we evaluated the expression of other surface antigens. As opposed to CD30, HLA-DR and PDL-1 relative transcript levels and cell surface expression were significantly reduced in R-cells. Intriguingly, BDM-induced CD30 upregulation was specific to L1236 cells since extended exposure to BDM did not modify expression levels of CD30 in other lymphoma cell types (SUDHL1, JEKO). BDM-induced CD30 overexpression significantly enhanced the sensitivity of HL cells to the cytotoxic effects of BV. MTS assay showed the IC50 of R100 cells to BV shifted to 0.21 ± 0.06 mcg/mL (48 hrs) and 0.19 ± 0.05 mcg/mL (72 hrs) vs. 3.16 ± 0.75 mcg/mL (48 hrs) and 3.87 ± 0.68 mcg/mL (72 hrs) of parental cells, a 15- and 20-fold increase, respectively (p<0.001; Figure 1B). Finally, qRT-PCR studies disclosed that R-clones did not show any upregulation of the drug exporter MDR1 gene, involved in MMAE extrusion and BV resistance in HL cells, as compared to parental L1236 cells.
Conclusions: Whileacute exposure to BDM induced a transient upregulation of CD30, extended exposure to the agent resulted in the selection of L1236 subclones with a stable overexpression of surface CD30 and reduced levels of HLA-DR and PDL-1. Upregulation of CD30 was associated with a significantly enhanced sensitivity to cytotoxic effects of BV. Translated into a clinical context these findings suggest that HL patients progressing upon BDM treatment may turn exquisitely sensitive to BV and prompt the exploration of a sequential treatment with BDM and BV in late and earlier disease phases. Results also provide a mechanistic insight as to the efficacy of the BDM-BV combination in HL, highlighting non-overlapping resistance pathways between BDM and MMAE. BDM-induced selection of CD30hi, HLA-DRlow/PDL-1low tumor cell clones might be relevant to the design of novel treatment strategies for HL based on the combination of BDM with BV and anti-PD1/PDL-1 antibodies.
Expression of CD30 and HLA-DR in parental (L1236) and BDM-resistant (R) HL cells
Expression of CD30 and HLA-DR in parental (L1236) and BDM-resistant (R) HL cells
Sensitivity to BV of parental (L1236) and BDM-resistant (R100) HL cells
Pinto:Takeda: Research Funding; Takeda, Celgene, Roche, TEVA: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal