Abstract
Background
The "double hit" (DH) lymphomas that harbor a c-myc mutation and BCL2 translocation, or "double protein expressor" (DP) lymphomas that over-express c-myc and BCL2 proteins in the absence of a detectable mutation, have amongst the worst clinical outcomes as compared to patients with diffuse large B-cell lymphomas (DLBCL) that lack upregulation of the c-myc oncogene. Metformin can down-regulate translation of c-myc, making it an appropriate anti-cancer drug to explore in c-myc+ lymphomas. Furthermore, amethod to identify DH/DP patients most likely to benefit from metformin treatment has clinical relevance.
Methods
Within a publicly available gene expression array data set of R-CHOP treated DLBCL (n=232; GSE10846), a subset of DH/DP patients were defined as having above median expression of myc and BCL2 and below median expression of BCL6 as previously published by Horn et al. Survival analysis, significance analysis of microarrays (SAM) and gene set analysis (GSA) were performed characterizing the clinical, individual gene and biological ontology differences between DH/DP and non-DH/DP populations. Expression array data from a study testing metformin effects on THP-1 monocyte cells was reanalyzed using SAM and GSA as well. Changes in individual gene expression and overlapping ontological themes common to both GSA analyses of metformin effects on THP-1 cells and DH/DP characterization were identified. Genes with differential expression (DE) in both groups were evaluated topologically using a protein-protein interaction database to determine if any gene products had previously observed direct interactions. Network community detection identified tightly coupled signaling modules linking co-expression to mechanism. The resulting metformin-DH/DP network metagene was evaluated by k-means, clustering tumor samples into two groups over the metagene members in an independent data set of R-CHOP treated DLBCL patients (n=249; GSE32918) with differences in overall survival (OS) determined by log-rank.
Results
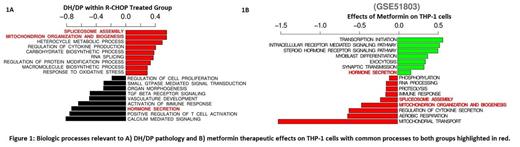
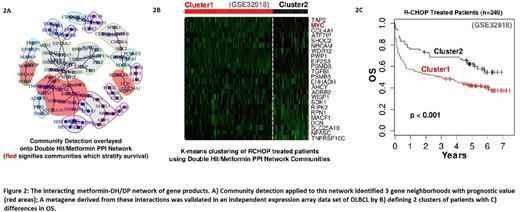
Of the 232 DLBCL patients treated with R-CHOP, 26 fit the criteria for DH/DP and had significantly lower OS (HR = 2.96; p < 0.001). In DH/DP tumors, 2780 genes had DE (2208 up-regulated; 572 down-regulated), enriched for biological processes related to transcription, metabolism and cytokine production and down-regulated for processes related to immune response, cell signaling, vascular development and proliferation (Fig. 1A). Analysis of metformin treated THP-1 cells relative to control identified 7123 genes with DE. Biological themes common to metformin treatment and DH/DP specific biology were identified including mitochondrial biogenesis, alternate splicing, and hormone secretion (Fig. 1A-B; highlighted in red). The intersection of genes with DE in metformin treated and DH/DP data sets identified 102 genes with direct interaction within a protein interaction network. Of the 19 communities detected by analyzing the resulting network topology, 3 showed significant correlation to survival in the GSE10846 data set (Fig. 2A, in red), forming a metformin-DH/DP metagene (Met-DH/DP-MG; n = 29 genes total). This metagene was validated by applying it to an independent cohort of R-CHOP treated DLBCL patients (n = 249), demonstrating 2 cluster groups (cluster 1, n=178; cluster 2, n=71; Fig. 2B) with differences in OS (HR = 1.61; p < 0.001; Fig. 2C).
Conclusion
We have identified a metagene of interacting proteins associated with both metformin therapeutic effect and OS in DH/DP patients. This offers a potential method for selecting patients most likely to benefit from metformin therapy and identifies mechanistic avenues by which metformin treatment may specifically benefit DH/DP patients. As such, in vitro studies using DH cell lines and a phase I/II clinical trial exploring chemo-immunotherapy with metformin as an adjunct in DH/DP lymphomas are underway.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal