Abstract
Background: Eculizumab was demonstrated to effectively treat and prevent thrombotic microangiopathy (TMA) and to improve renal function and hematologic parameters for up to 2 years in4 previous prospective clinical trials and in 1 retrospective study of patients with atypical hemolytic uremic syndrome (aHUS). Patients in these studies enrolled in a long-term follow-up study, in which rates of TMA were evaluated during eculizumab treatment and during discontinuation from eculizumab.
Methods: Data were gathered from an ongoing, observational, multicenter study of aHUS patients treated with eculizumab in 5 prior clinical studies. The primary endpoint was TMA event rate post-parent study during on-treatment (ON; among patients receiving eculizumab) and off-treatment (OFF; among patients who discontinued eculizumab) periods.
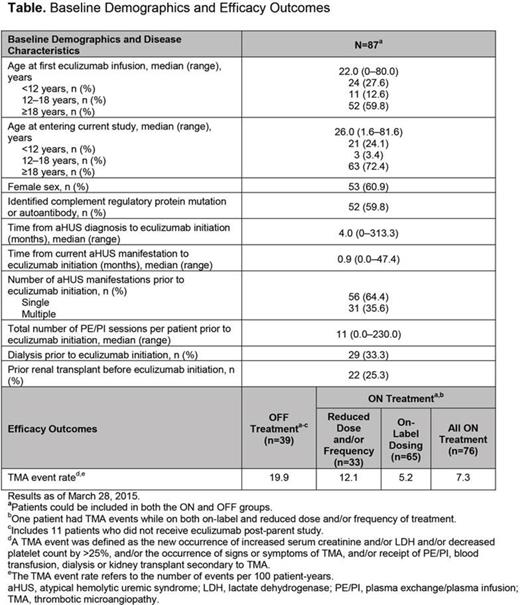
Results: As of March 28, 2015, 87 patients, including 35 children <18 years of age (40%) and 52 adults ≥18 years of age (60%), enrolled in the study (Table). Of these, 76 patients had ON and 39 patients had OFF treatment periods with median follow-up of 26.1 and 20.1 months, respectively. A reduced dosage of eculizumab was received by 33 of 87 patients (38%). Seventeen of 39 patients (44%) reinitiated eculizumab after therapy discontinuation. The TMA event rates were 7.3 and 19.9 per 100 patient-years for ON and OFF patients, respectively. The rate of TMA in ON patients was greater in those receiving reduced dosing compared with label recommendations (Table). OFF patients had higher estimated glomerular filtration rates at time of discontinuation than ON patients. Age, frequencies of higher-risk complement factor mutations (CFH, C3, or CFB) and kidney transplant status were not different between ON and OFF patients. Factors that independently predicted TMA events included: ON treatment status (compared with OFF; hazard ratio [HR], 0.19; 95% confidence interval [CI], 0.07‒0.49; P<0.001), higher-risk mutations (compared with no mutation; HR, 5.51; 95% CI, 1.61‒18.84; P=0.007), and lower-risk mutations (compared with no mutation; HR, 3.70; 95% CI, 1.05‒13.02; P=0.04).
Conclusions: The TMA event rate was 2.7-fold higher after eculizumab discontinuation compared with patients receiving ongoing eculizumab therapy. TMA event rates were lowest during on-label dosing, higher during reduced dosing, and highest during off-treatment periods. These findings suggest that patients with aHUS have a progressive increase in the risk of TMA events during periods of reduced dosing and after discontinuation of eculizumab, compared with on-label eculizumab dosing.
Greenbaum:Alexion Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Fakhouri:Alexion Pharmaceuticals: Consultancy. Kincaid:Alexion Pharmaceuticals: Employment, Equity Ownership. Licht:Alexion Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Achillon: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Mix:Alexion Pharmaceuticals: Employment, Equity Ownership. Provôt:Alexion Pharmaceuticals: Honoraria. Rondeau:Alexion Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees. Sheerin:Alexion Pharmaceuticals: Honoraria. Wang:Alexion Pharmaceuticals: Employment, Equity Ownership. Menne:Alexion Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal