Abstract

Results from cooperative group trials demonstrating the effectiveness of arsenic trioxide in the treatment of newly diagnosed APL have thus far been restricted to trials consisting of primarily adult patients. The Children's Oncology Group trial AAML0631 incorporated two cycles of arsenic trioxide (ATO) during consolidation with approximately 40% reduction in anthracycline dosing compared to the Italian AIDA0493 trial which has the largest cohort and best phase III results published for pediatric APL (Testi et al Blood 2005).
Eligibility criteria included age ≥ 2 to < 22 years, de novo APL confirmed by PML-RARA PCR and no prior therapy. Diagnostic white blood cell count (WBC) determined risk group as standard risk (SR) for WBC < 10,000 and high risk (HR) for WBC ≥ 10,000. ATRA was given at the pediatric dose of 12.5 mg/m2/dose PO BID on days 1-30 of induction and days 1-14 of each consolidation course and each maintenance cycle. Other therapy was as follows: Induction- idarubicin 12 mg/m2/dose for 3 doses; Consolidation 1- 2 cycles of ATO 0.15 mg/kg/day IV 5 days each week for 5 weeks; Consolidation 2- cytarabine 1,000 mg/m2/dose IV q12 hours days 1-3, mitoxantrone 10 mg/m2/dose IV days 3, 4; Consolidation 3- idarubicin 5 mg/m2/dose IV days 1, 3, 5; Consolidation 4 (HR APL only)- cytarabine 1,000 mg/m2/dose IV q12 hours days 1-3, idarubicin 10 mg/m2/dose IV day 4; Maintenance (12 weeks/cycle for 9 cycles)- 6-mercaptopurine 50 mg/m2/day PO, methotrexate 25 mg/m2 PO once weekly. Intrathecal cytarabine was given once during each consolidation 2-4 and cycle 1 of maintenance.
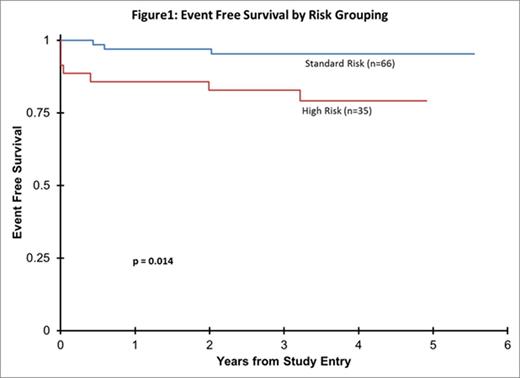
Between 3/2009 and 11/2012, 108 patients enrolled and 101 (66 SR and 35 HR) were evaluable for outcome (4 PML-RARA PCR negative, 3 local consent issues). Hematologic CR after induction (without central review) was 81%. The molecular (RQ-PCR) remission rate for those tested at end of Consolidation was 100%. For all patients, overall survival (OS) was 94% and event free survival (EFS) was 92% at 2 years. Comparing SR to HR, the OS was 98% vs. 86% (p=0.004) and EFS was 97% vs. 83% (p=0.014) (Figure 1). The predetermined statistical plan was to compare the EFS results to AIDA0493 which had 2 year EFS of 91% for SR and 79% for HR APL. Relapse risk on AAML0631 from end consolidation I (following ATO treatment) was 2% at 2 years and did not differ significantly between patients with SR vs. HR APL.
Events on this trial included 3 relapses, 1 second malignant neoplasm (squamous cell carcinoma in situ) and 7 deaths (6 HR APL, 1 SR APL) including one death post-relapse. Four deaths during induction (all HR APL) included multiple etiologies, but coagulopathy was a factor in each. There were 2 post-induction on-therapy deaths: SR APL patient with accidental (non-chemotherapy) drug overdose during Consolidation 3 and HR APL patient with gram negative sepsis during Consolidation 2. Three relapses included: combined molecular bone marrow (detected by PCR) and CNS relapse in a SR APL patient during maintenance cycle 7 (24 months post diagnosis), combined hematologic bone marrow and CNS relapse in a HR APL patient during maintenance cycle 6 (23 months post diagnosis), and molecular bone marrow relapse in a HR APL patient after therapy completion (38 months post diagnosis) who later died of stem cell transplant complications. Both patients with molecular relapse had low level PML-RARA transcript detection (under 0.001 NCN) during maintenance beginning 1 and 16 months prior to relapse. No other patients had similar "borderline" results. While 28 patients had a diagnostic LP performed in pre-therapy evaluation, 7 patients met the protocol definition of CNS disease (CNS2 N=4, or CNS3 N=3). The 2 patients with relapse involving the CNS did not have CNS disease at diagnosis.
Historically, AIDA0493 represents the largest (N=107) and best published outcomes for a major pediatric phase III trial in APL. The current trial of 101 pediatric patients achieved higher EFS rates in both SR and HR APL through incorporation of ATO while significantly reducing anthracycline doses. The reported CR rate was lower than historical comparisons, but this was likely due to difficulty in assessing differentiating and recovering bone marrow cells after APL induction. The excellent molecular remission rate at end of consolidation and the low relapse rate confirm the effectiveness of this therapy. These results strongly support use of ATO in pediatric patients with newly diagnosed APL.
Off Label Use: Arsenic Trioxide in pediatric patients with newly diagnosed APL. Gemtuzumab Ozogamicin in Pediatric AML..
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal