Abstract
Background: Anticancer therapeutics leverages activation of apoptosis signal transduction pathways (extrinsic and intrinsic apoptotic pathways) in cancer cells. Apoptosis induced by the extrinsic pathway complements that induced by the intrinsic pathway, so targeting extrinsic pathway is considered a useful new therapeutic approach. Preclinical data suggests TNF related apoptosis inducing ligand (TRAIL) as a promising approach as apoptosis of tumor cells is achievable in vivo without lethal toxicities. CASP8 and FADD-like apoptosis regulator (CFLAR) is an inhibitor of death receptor signaling that inhibits TRAIL-mediated caspase 8 auto-activation and subsequent apoptosis. We recently identified a splicing single nucleotide polymorphism (SNP) rs10190751 G>A in CFLAR, where presence of the variant allele (A) was associated with alternate splicing as well as with chemo-sensitivity to chemotherapeutic agent triptolide. However role of CFLAR and the splicing SNP on chemo-sensitivity to wide array of anticancer drugs is not known.
Objective: Given the central role of CFLAR in apoptotic pathway, the goal of this study was to investigate impact of CFLAR and its splicing SNP on cytotoxicity of wide range of chemotherapeutic drugs including the ones extensively used in hematological malignancies.
Methods: We selected chemotherapeutic agents with wide range of mechanisms of action as blocking DNA biosynthesis, interfering with structure or function of DNA or protein synthesis, interfering with DNA transcription or replication as well as drugs that are cell cycle specific or not. We selected nine Epstein-Barr-virus transformed lymphoblastoid cell lines (LCLs) that are part of International HapMap project representing different genotype for rs10190751 (CFLAR splicing polymorphism; 3 in each genotype category) with twelve different chemotherapeutic agents. Further validation of CFLAR's role in in vitro chemosensitivity was evaluated using CFLAR knockdown and overexpression studies in pancreatic and leukemic cell lines such as Panc-1 and THP1.
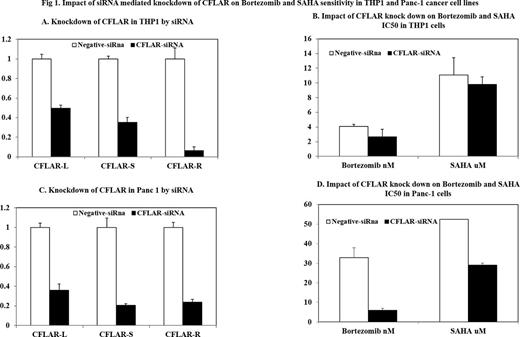
Results: CFLAR splicing SNP rs10190751, was associated with in vitro cytotoxicity of several chemotherapeutic agents (Bortezomib, SAHA, doxorubicin, sorafenib). The results of screening of 122 FDA approved drugs and their relation with CFLAR as well as its splicing SNP will be presented at the annual meeting. As an example we show below that knock down of CFLAR isoforms have a significant impact on in vitro chemosensitivity to bortezomib and SAHA (Figure 1)
Conclusion: Our results suggest critical role of CFLAR in anticancer drug mediated cell death. Additionally splicing SNP in CFLAR seems to play an important role in drug sensitivity/resistance. Therapeutic strategies to directly or indirectly inhibit the expression and/or function of CFLAR might be an attractive option to overcome resistance to wide range of chemotherapeutic agents.
Impact of siRNA mediated knockdown or of CFLAR on Bortezomib and SAHA sensitivity in THP1 and Panc-1 cancer cell line.
Impact of siRNA mediated knockdown or of CFLAR on Bortezomib and SAHA sensitivity in THP1 and Panc-1 cancer cell line.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal