Abstract
Introduction
Regulatory T cells (CD4+CD25high CD127low Foxp3+) (Tregs) are of increasing interest in the context of allogeneic hematopoietic cell transplantation (HCT), as they may be able to prevent or suppress graft-versus-host disease (GVHD). Relatively little is known about Treg profiles during the post-HCT period. We tested the hypothesis that Treg phenotype and function correlates to GVHD status.
Methods
Peripheral blood was drawn at 100 days and six months post-HCT from adult patients enrolled on an IRB-approved protocol. Eight-color flow cytometric analysis of Treg subsets was performed on peripheral blood mononuclear cells. Patients' FACS-sorted CD4+CD25high CD127low CD49dlow Tregs were cultured with their CD3/CD28-stimulated T cells and analyzed for proliferation via 3H-thymidine incorporation and cytokine production in culture supernatants. Clinical GVHD evaluations were extracted from medical records. Age-matched healthy controls were recruited for comparison. Data were analyzed using univariate regression, Mann-Whitney t-tests, ANOVA, and Wilcoxon matched-pairs signed rank tests.
Results
Twenty-eight patients (median age 61 years old) were studied. Total percentages of Tregs were similar at +100d and +6m post-HCT (median 9.3% and 9.15% of total CD4+ T cells, respectively), with similar levels of transcription factor Helios and markers of memory/activation (CD45RO), proliferation (Ki-67), and apoptosis (activated caspase-3). Healthy controls' Tregs had higher caspase-3 expression than patients'; all other markers exhibited similar levels. CD45RO+ and Ki-67+ levels were increased among Helios+ Tregs compared to Helios-Tregs, whereas caspase-3 levels were decreased. The absolute number of circulating Tregs was comparable between +100d and +6m, displaying lower numbers compared to healthy controls attributable to overall lower total T cell numbers.
In vitro addition of sorted Tregs to stimulated bulk T cells isolated from patients at +100d post-HCT induced median 41% proliferation reduction (i.e., suppression), reduced levels of pro-inflammatory IL-2, IL-5, GM-CSF, IFNγ, and TNFα, and increased levels of anti-inflammatory IL-10 compared to stimulated bulk T cells alone (Treg:T bulk ratio of 1:2). These changes were of equivalent magnitude in cultures from healthy controls. No net change was noted for IL-17, while IL-35 and IL-12 levels were below detection. Univariate analysis revealed linear relationships between cellular proliferation suppression and IL-2, IL-4, IL-10, IL-13, GM-CSF, IFNγ, and TNFα relative changes.
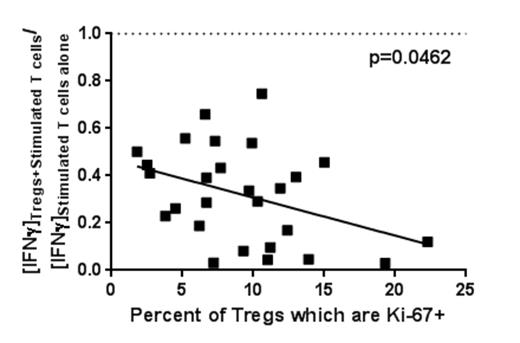
Across all +100d samples, higher degrees of Treg proliferation (Ki-67+) corresponded with increased IFNγ and TNFα suppression in culture supernatants (p=0.0462 and p=0.0276, respectively) and specifically correlated with enhanced suppression of IFNγ-producing CD8+ T cells (p=0.0280). Furthermore, higher Treg expansion potential (measured by Treg Ki-67/caspase-3 ratio) suppressed IFNγ and TNFα more potently (p=0.0479 and p=0.0484, respectively) and was associated with a higher proportion of Tregs comprising total CD4+ T cells (p=0.0266).
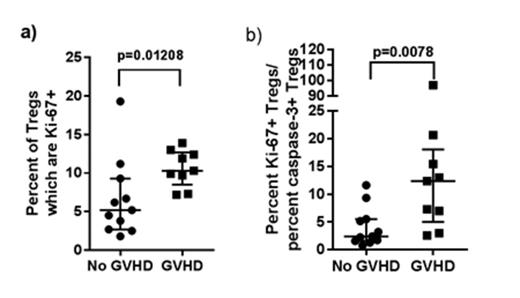
Higher Treg Ki-67 expression at 100 days post-HCT was associated with increased likelihood of de novo GVHD onset by six months post-HCT (p=0.01208). Similarly, a higher Treg expansion potential profile was associated with GVHD onset by six months (p=0.0078).
Conclusion
Tregs from patients at 100 days and six months post-HCT display similar memory/activation and proliferation profiles and functional suppressive ability as Tregs from healthy controls. Among these patients, a proliferative Treg profile strongly correlates with increased in vitro suppressive function on a cell-per-cell basis and is further associated with reduced apoptotic profile, representing an expansive Treg profile. As these profiles can predict GVHD developing by six months post-HCT, the increased associated cell-per-cell suppressive potential may represent a compensatory yet ultimately inadequate Treg response to early GVHD-associated inflammatory changes.
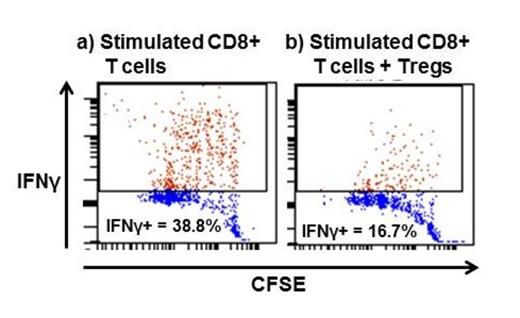
In vitro CD8+ IFNγ production decreases after Treg addition (representative diagram).
In vitro CD8+ IFNγ production decreases after Treg addition (representative diagram).
Increased Treg proliferative profile (Ki-67+) is associated with greater in vitro IFNγ suppression.
Increased Treg proliferative profile (Ki-67+) is associated with greater in vitro IFNγ suppression.
Increased Treg (a) proliferative and (b) expansion potential profiles at 100 days post-HCT predict de novo GVHD by six months.
Increased Treg (a) proliferative and (b) expansion potential profiles at 100 days post-HCT predict de novo GVHD by six months.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal