Abstract
Introduction. The viral oncolytic agent, Reolysin (RV), is a promising novel therapeutic that selectively proliferates in myeloma cells. Our group conducted a phase 1 clinical trial of single agent RV in patients with relapsed and refractory multiple myeloma (MM), and reported that treatment was well tolerated and associated with prolonged disease stability in 25% of patients. Objective responses were not evident, likely because the viral RNA present in the myeloma cells was not producing infectious viral particles. Proteasome inhibitors can lead to myeloma cell death due to increased endoplasmic reticulum (ER) stress and induction of ER-stress related apoptosis (Kelly, Oncogene, 2012). We confirmed this effect preclinically with Carfilzomib (CFZ), and hypothesized that the addition of CFZ to RV would increase viral proliferation and MM cell death sufficiently to obtain objective response in patients with relapsed MM.
Methods. For this pilot trial, patients were required to have relapsed myeloma with IMWG-defined measurable disease, ANC ≥ 1,000/uL, platelet count ≥ 50,000/uL, with no creatinine requirements. Cohorts of 6 patients each were planned. Cohort 1 included patients who were CFZ na•ve or had not progressed on a CFZ containing regimen. Intravenous CFZ (20 mg/m2 days 1 and 2 of cycle 1 and 27 mg/m2 thereafter), Reolysin (3 x 1010 TCID50/day), and dexamethasone (20 mg) were administered on days 1, 2, 8, 9, 15, and 16 of a 28-day cycle (Table 1). In situ based methodologies were used to examine the distribution of CD138, CD8, NK cells (CD117 and IL-22), CD 68, PD L1, reoviral capsid protein, and reoviral RNA in bone marrow biopsies performed prior to treatment on days 1 and 9 of cycle 1.
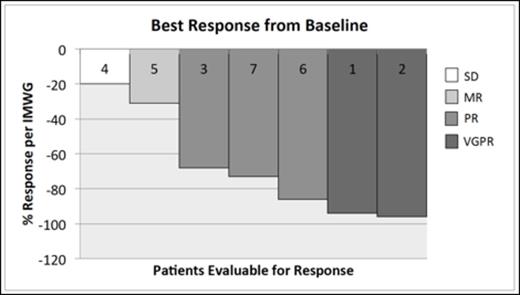
Results. Seven patients have been enrolled, four are male, and all are Caucasian. Patients have a median age of 64, and have received on average 2.4 prior lines of therapy and 4.4 prior treatments. All patients were previously exposed to Revlimid and Velcade, and 4 patients were Velcade refractory. One patient was previously treated with CFZ but was deemed to be CFZ sensitive, one patient has dialysis-dependent CKD, and all but one patient had evidence of high-risk cytogenetics on CD138-selected FISH at the time of enrollment. 6/7 patients suffered myalgias and fever after the first two doses of Reolysin, but these symptoms did not recur in any subsequent doses. Treatment has been well tolerated in 5 patients, but 2 patients were removed from study after 2 doses of combination therapy, one for congestive heart failure, and the other for gastrointestinal bleed in the setting of grade 4 thrombocytopenia and an arteriovenous malformation. Due to these 2 DLTs, patient 7 was enrolled at dose level -1 (Carfilzomib 20 mg/m2 and Reolysin 3 x 109 TCID50/day on days 1, 2, 8, 9, 15, and 16 of a 28 day cycle). Within the first 14 days following the initiation of treatment, the mean decrease in platelets for the 7 evaluable patients was 79 (50 - 139), and this included grade 4 (N = 1), and asymptomatic grade 2 (N = 3), and grade 1 (N = 3) events. All patients have had a reduction of the monoclonal protein, 5 patients remain on study, and the longest duration of response is currently 8 cycles. Responses are VGPR (N = 2), PR (N = 3), MR (N = 1), and SD (N = 1) (Figure 1). Intracellular viral replication will be reported at the meeting.
Conclusion. This 3-drug regimen is relatively well tolerated in heavily treated patients with relapsed MM. Most patients experience low grade fever and myalgias after the first two doses, and patients have evidence of thrombocytopenia in cycle 1. Combination treatment is associated with reduction of the monoclonal protein in all patients, and 86% (6/7) CFZ-sensitive patients have evidence of objective response.
Combination treatment dose levels
| Dose level . | Dexamethasone (IVP) . | Carfilzomib (IVPB) . | Reolysin (IVPB) . |
|---|---|---|---|
| -1 | 20 mg/day | 20 mg/m2 /day | 3 x 109 TCID50/day |
| 1 (starting dose) | 20 mg/day | C1 Day 1 & 2 - 20 mg/m2 /day C1 Day 8 & onward - 27 mg/m2 /day | 3 x 1010 TCID50/day |
| Dose level . | Dexamethasone (IVP) . | Carfilzomib (IVPB) . | Reolysin (IVPB) . |
|---|---|---|---|
| -1 | 20 mg/day | 20 mg/m2 /day | 3 x 109 TCID50/day |
| 1 (starting dose) | 20 mg/day | C1 Day 1 & 2 - 20 mg/m2 /day C1 Day 8 & onward - 27 mg/m2 /day | 3 x 1010 TCID50/day |
Waterfall plot representing response of 7 patients with relapsed MM
Off Label Use: Reolysin - oncolytic viral, anti-cancer agent.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal