Abstract
Telomeres are nucleo-protein complexes at the ends of the chromosomes that play a key role in protection of the ends from being recognized as DNA damage and to prevent fusion of the chromosomes. The telomeric DNA shortens with each cell division in the absence of telomerase, due to end replication problem. In chronic lymphocytic leukemia (CLL), short telomeres were found to be associated with poor prognostic factors and poor survival in various univariable and multivariable analyses. Short telomeres in CLL are known to be frequently associated with increased DNA damage response and to undergo fusion events, conferring genomic instability. But the contribution of telomere dysfunction to CLL pathogenesis and disease progression has never been studied in vivo using mouse models.
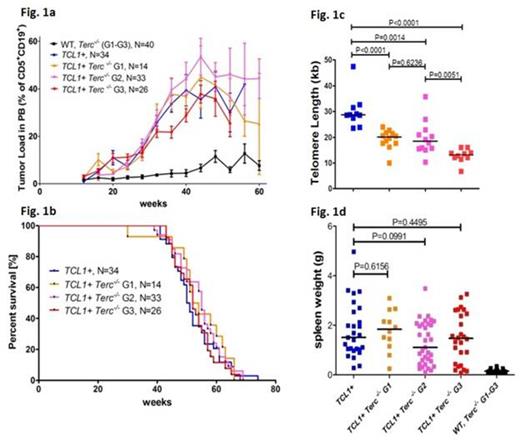
Here, we hypothesized that genomic instability resulting from telomere dysfunction could drive acquisition of genetic lesions, contributing to CLL pathogenesis, progression and disease evolution. Thus, the CLL mouse model with telomere dysfunction was generated by crossing the Eµ-TCL1 (TCL1+) mouse with mTerc-/- mouse. The first generation TCL1+ mTerc-/- (G1) mice were inter-crossed to obtain generations G2 and G3, as telomeres are known to shorten with subsequent generations. The TCL1+ mTerc-/- mice from the generations G1 (N=14), G2 (N=33) and G3 (N=26), including TCL1+ (N=34), wildtype (WT, N=18) and mTerc-/- G1 (N=4), G2 (N=5) and G3 (N=13) as controls were initially analyzed for disease burden in peripheral blood (PB) by bleeding at an interval of 4 weeks, starting from 12 weeks and the percentage of CD19+ CD5+ cells was estimated by FACS. No difference in disease onset or progression was observed between the TCL1+ mTerc-/- G1, G2 and G3 in comparison toTCL1+ mice (Fig. 1a). Similarly, analysis of survival showed no significant difference between the TCL1+ mTerc-/- G1 (N=14), G2 (N=33) and G3 (N=26) mice, compared to TCL1+ (N=34) (median: 53, 55, 52 weeks vs. 50.5 weeks, Fig. 1b).
Spleen and liver weights in the TCL1+ mTerc-/- G1 (N=12), G2 (N=33) and G3 (N=26) mice were highly variable (spleen: 0.1g to 3.5g, liver: 0.1g to 8.0g) as in the TCL1+ (N=27, spleen: 0.3g to 5.0g, liver: 1.7g to 7.4g) mice but no significant difference in spleen (Fig. 1d) and liver weights was observed between the subgroups. Interestingly, spleen weights were associated with survival only in the TCL1+ mice, with larger spleens associated with worse survival (48.5 vs. 57.5 weeks, P=0.091). Since no difference in disease characteristics was observed, it was verified using Q-PCR, if telomere lengths vary in the tumors from the different subgroups. Telomere lengths of CLL cells from the spleen were significantly shorter (Fig. 1c) in the G1 (median: 20.5kb, P=0.0002), G2 (median: 18.5kb, P=0.0016) and G3 (median: 13.2kb, P<0.0001) compared to TCL1+ (median: 28.7kb). The absence of correlation of telomere length with survival in the murine CLL models with telomere dysfunction may indicate that a critical telomere length in the tumor is yet to be reached to elicit genetic alterations and clonal selection.
Additionally, the G3 mTerc-/- microenvironment is known to restrict B and T lymphopoiesis and thus might influence CLL cell proliferation, masking disease aggressiveness in the TCL1+ mTerc-/- G3 mice. To overcome the influence of mTerc-/- microenvironment, CLL cells obtained from spleens of TCL1+ and TCL1+ mTerc-/- G3 mice were transferred into syngeneic C57Bl6 mice. Briefly, 20 million cells were intravenously injected into the tail vein and disease was monitored by analysis of CD19+ CD5+ cells in PB, once every 4 weeks. Early follow up of 8 weeks clearly show a trend towards increase in CLL cells in PB of mice transferred with TCL1+ mTerc-/- G3 tumors compared to those with TCL1+ tumors (median tumor load: 15.75% vs. 6.1%, P=0.0553). Longer follow up of the experiment is ongoing.
In summary, the TCL1+ mTerc-/- mice across the generations G1, G2 and G3 showed no difference in disease onset, progression, disease burden and survival in comparison to TCL1+ mice. The absence of increased disease manifestation in the TCL1+ mTerc-/- may be attributed to the microenvironmental influence on lymphopoiesis, as syngeneic transfer of CLL from TCL1+ mTerc-/- G3 mice showed an increase in tumor load compared to that of TCL1+ tumors, indicating a contribution of telomere shortening to disease aggressiveness in CLL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal