Abstract
BACKGROUND: Minimal residual disease (MRD) in CLL is an independent predictor of progression-free and overall survival after chemo-immunotherapy. Data from the DCLLSG trials indicate that a high, intermediate and low risk of disease progression is seen in patients with >1%, 0.01-1%, or <0.01% MRD respectively. The survival benefit per log reduction is informative in other disorders and may be a more informative measure for comparing different treatments.
AIM: To apply the ERIC consensus 1-tube multiparameter MRD strategy prospectively in two UK clinical trials of FCR-based treatment (ADMIRE and ARCTIC) to determine the survival benefit per log depletion.
METHODS: The level of residual disease was determined using multi-parameter flow cytometry according to the ERIC consensus protocol with a limit of quantification of 10-4 / 0.01% or better on 415 patients at 3 months after end of treatment.
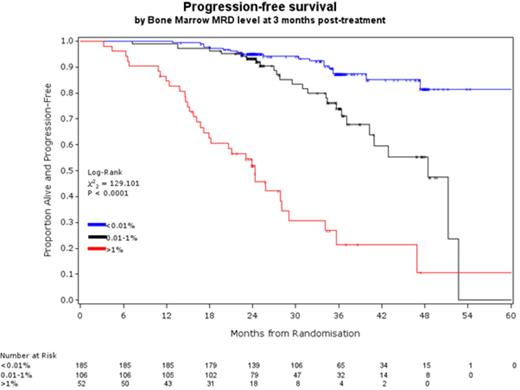
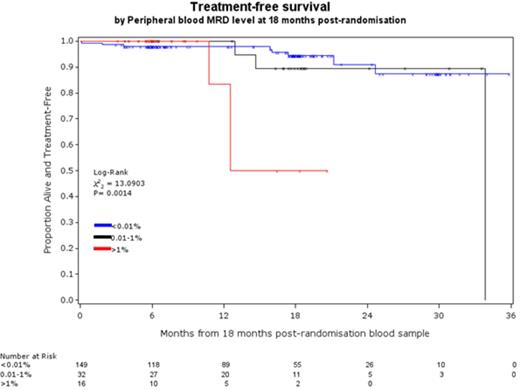
RESULTS: The level of MRD at end of treatment was a powerful predictor of PFS and OS independent of age, stage, IWCLL response, FISH and IGHV mutation status. Per log reduction in CLL level, the hazard of disease progression decreased by 33% (95%CI 27-38%) and the hazard of dying decreased by 22% (95%CI 13-29%). Although there were statistically significant improvements per log reduction, the DCLLSG 2-log model showed more meaningful differences in outcome over the period of follow-up. The median PFS for patients with >1% vs. 0.01-1% vs <0.01% BM MRD was 24 months vs. 48 months vs. not reached (NR, 82% progression-free at 48 months, figure 1a) respectively and the median OS was 49 months vs. NR (78% alive at 48M) vs. NR (85% alive at 48M) respectively. Median PFS and OS for all patients achieving a PR or worse was 41M and 51M respectively indicating that the presence of >1% MRD predicts equivalent or worse outcome than a clinical PR. PB MRD analysis at 18 months after randomisation (~1 year after treatment) was also strongly predictive of outcome: 98% of patients with <0.01% PB CLL at the 18M timepoint remain alive and treatment-free for the subsequent year (Figure 1b).
CONCLUSIONS: Prospective enumeration of MRD using the ERIC consensus 1-tube multiparameter protocol confirms that MRD at end of treatment is a powerful independent predictor of progression-free and overall survival. Post-treatment follow-up using peripheral blood MRD could be more informative than clinic assessments because patients with <0.01%MRD are highly unlikely to require treatment within the following year while in patients with ≥0.01%MRD the rate of disease progression can be accurately predicted. Patients with >1% MRD at end of treatment in the peripheral blood have a similar outcome to those with a clinical PR and a bone marrow assessment is not informative in these cases. The level of MRD is highly informative with sequential improvements in outcome per log depletion. The DCLLSG 2-log categorisation (>1%, 0.01-1%, or <0.01%) is simple and effective for discriminating patients with PFS of <2yrs vs. >6yrs.
the level of MRD in the bone marrow at 3 months after treatment is highly predictive of outcome with >1% MRD equating to <2yrs PFS and <0.01% MRD predicting >5yrs median PFS
the level of MRD in the bone marrow at 3 months after treatment is highly predictive of outcome with >1% MRD equating to <2yrs PFS and <0.01% MRD predicting >5yrs median PFS
sequential PB MRD analysis identifies patients with negligible risk of requiring treatment within the following 12 months.
sequential PB MRD analysis identifies patients with negligible risk of requiring treatment within the following 12 months.
Rawstron:BD Biosciences: Patents & Royalties; Roche: Honoraria; Celgene: Honoraria; Abbvie: Honoraria; Gilead: Honoraria, Research Funding; Pharmacyclics: Research Funding. Gregory:Celgene: Honoraria; Janssen: Honoraria. Hillmen:Roche: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal