Abstract
Introduction: Transfusional hemosiderosis is common in myelodysplastic syndromes (MDS). There are multiple retrospective analyses demonstrating a survival benefit associated with iron chelation therapy (ICT) in lower risk, transfusion dependent (TD) MDS patients. However, these studies are limited by their retrospective nature, potential for bias and by the use of risk scores at diagnosis rather than at the onset of TD. Since January 2012 the Canadian MDS Registry has prospectively collected disease and patient-related data on MDS patients including comorbidity (Charlson and MDS-CI), frailty (Rockwood clinical frailty scale) and disability [Lawton Brody Instrumental Activities of Daily Living (sIADL)]. We compared characteristics and clinical outcomes of lower risk TD MDS patients who received ICT to non chelated TD patients, adjusting for MDS and patient-related factors.
Methods: Only patients who remained International Prognostic Scoring System (IPSS) low or intermediate (int)-1 risk at the time of first TD were included with MDS and patient-related factors analyzed at first TD rather than at MDS diagnosis or registry enrollment. Univariate and multivariate Cox proportional hazard models were used to determine significant predictive factors for overall survival (OS) and the model with the highest R2 was selected.
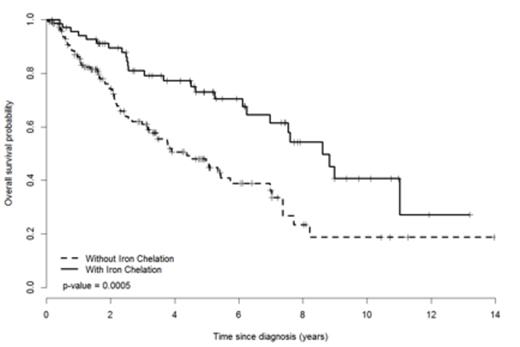
Results: 219 Low (n=69)/Int-1 (n=149) risk MDS patients at the time of first TD were included. Median age was 73 [interquartile range (IQR 65,80)] with a median time from diagnosis until TD of 7 months (IQR 1,28). 60% were male with a median ECOG of 1 and median blast of 3% (IQR 1,4). By WHO classification, 39% and 37% had unilineage and multilineage dysplasia respectively, 11% CMML and 13% had excess blasts. By IPSS-revised (R), very low, low, intermediate, high and very high risk groups were 12%, 34%, 38%, 15% and 0.5%, respectively. Seventy (32%) patients received ICT with desferrioxamine (n=6), deferasirox (n=56) or both (n=8). At the time of first TD, chelated patients were younger, had higher ferritins and had lower IPSS-R risk scores (Table 1). Importantly, frailty, comorbidity, and disability scores did not differ. At a median follow up of 2.7 (IQR 2.2-3.3) years from diagnosis, OS was 6.1(IQR 4.5-7.5) years. OS was significantly improved among MDS patients treated with ICT as compared to those without (median 8.62 vs. 4.38 years, respectively, p = 0.0005, Figure 1.) By univariate analysis, age, ICT, IPSS, IPSS-R, MDS-CI, frailty, karyotype, time from diagnosis until TD, and disability were associated with improved OS. By multivariate analysis, ICT, age at TD and IPSS-R at TD were independently predictive of OS (Table 2).
Conclusions: Adjusting for patient and disease related factors at the time of TD, ICT remains predictive of improved OS in patients with low/int-1 risk MDS who become TD. The adjustment for patient-related factors and analysis from TD rather than MDS diagnosis, diminishes the impact of selection bias that may have favored ICT patients in past analyses and lends additional support to the role for ICT in lower risk MDS.
Comparing Clinical Factors at the time of First Transfusion Dependency (TD) between Chelated and Non-Chelated Patients
| Factors at Time of TD Median (IQR) . | Without Iron Chelation (n=149) . | With Iron Chelation (n=70) . | p-value . |
|---|---|---|---|
| Age (y) | 75 (67,81) | 69 (62,75) | 0.0008 |
| Ferritin (ug/L) | 664 (346,1118) n=92 | 1201 (883,1691) n=44 | <0.0001 |
| RA/RARS, del5q, MDS-U, RCUD [N(%)] RCMD+/-RS CMML/MDS/MPN RAEB1 RAEB2 | 51 (34) 56(38) 18 (12) 17 (11) 7 (4) | 35 (50) 25 (36) 3 (4) 6(9) 1(1) | 0.11 |
| IPSS [N(%)] Low Int-1 | 42 (28) 106 (72) | 27 (39) 43 (61) | 0.16 |
| IPSS-R [N(%)] Very Low Low Intermediate High Very High | 15 (10) 48 (32) 54 (36) 30 (20) 1 (0) | 12 (17) 26 (37) 29 (41) 3 (4) 0 (0) | 0.01 |
| Frailty | N=96 3 (2,4) | N=37 3 (2,4) | 0.40 |
| Charlson Comorbidity | N=95 1 (0,2) | N=37 0(0, 1) | 0.06 |
| MDS-CI | N=95 1 (0,2) | N=37 0 (0,2) | 0.26 |
| Lawton Brody Disability | N=90 1 (0,2) | N=36 0 (0,2) | 0.68 |
| Time from diagnosis until TD (mo) | 6 (1,23) | 14 (0,38) | 0.19 |
| Factors at Time of TD Median (IQR) . | Without Iron Chelation (n=149) . | With Iron Chelation (n=70) . | p-value . |
|---|---|---|---|
| Age (y) | 75 (67,81) | 69 (62,75) | 0.0008 |
| Ferritin (ug/L) | 664 (346,1118) n=92 | 1201 (883,1691) n=44 | <0.0001 |
| RA/RARS, del5q, MDS-U, RCUD [N(%)] RCMD+/-RS CMML/MDS/MPN RAEB1 RAEB2 | 51 (34) 56(38) 18 (12) 17 (11) 7 (4) | 35 (50) 25 (36) 3 (4) 6(9) 1(1) | 0.11 |
| IPSS [N(%)] Low Int-1 | 42 (28) 106 (72) | 27 (39) 43 (61) | 0.16 |
| IPSS-R [N(%)] Very Low Low Intermediate High Very High | 15 (10) 48 (32) 54 (36) 30 (20) 1 (0) | 12 (17) 26 (37) 29 (41) 3 (4) 0 (0) | 0.01 |
| Frailty | N=96 3 (2,4) | N=37 3 (2,4) | 0.40 |
| Charlson Comorbidity | N=95 1 (0,2) | N=37 0(0, 1) | 0.06 |
| MDS-CI | N=95 1 (0,2) | N=37 0 (0,2) | 0.26 |
| Lawton Brody Disability | N=90 1 (0,2) | N=36 0 (0,2) | 0.68 |
| Time from diagnosis until TD (mo) | 6 (1,23) | 14 (0,38) | 0.19 |
Predictive factors for Overall Survival by Multivariate Analysis
| Predictive Factors . | p-value . | HR . | 95% CI of HR . | R2 (%) . | |
|---|---|---|---|---|---|
| Iron chelation (no vs. yes) | 0.0152 | 1.821 | 1.122 | 2.953 | 14.76 |
| Age at time of diagnosis (yr) | 0.0125 | 1.025 | 1.005 | 1.045 | |
| IPSS-R at time of TD | 0.0018 | ||||
| High/vHigh vs. Low | 0.0004 | 2.866 | 1.601 | 5.132 | |
| Int. vs. Low/vLow | 0.0775 | 1.523 | 0.955 | 2.429 | |
| High/vHigh vs. Low | 0.0292 | 1.882 | 1.066 | 3.322 | |
| Predictive Factors . | p-value . | HR . | 95% CI of HR . | R2 (%) . | |
|---|---|---|---|---|---|
| Iron chelation (no vs. yes) | 0.0152 | 1.821 | 1.122 | 2.953 | 14.76 |
| Age at time of diagnosis (yr) | 0.0125 | 1.025 | 1.005 | 1.045 | |
| IPSS-R at time of TD | 0.0018 | ||||
| High/vHigh vs. Low | 0.0004 | 2.866 | 1.601 | 5.132 | |
| Int. vs. Low/vLow | 0.0775 | 1.523 | 0.955 | 2.429 | |
| High/vHigh vs. Low | 0.0292 | 1.882 | 1.066 | 3.322 | |
Kaplan-Meier Curve of Overall Survival of Chelated and Non-Chelated Patients
Leitch:Alexion: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Exjade: Speakers Bureau. Wells:Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Alexion: Honoraria, Research Funding. Nevill:Celgene: Honoraria. Zhu:Novartis Canada: Membership on an entity's Board of Directors or advisory committees; Celgene Canada: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Yee:Oncoethix: Research Funding; Novartis Canada: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene Canada: Membership on an entity's Board of Directors or advisory committees, Research Funding. Leber:Celgene Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees. Sabloff:Celgene: Honoraria. Kumar:Celgene Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees. Geddes:Celgene: Honoraria. Storring:Celgene Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kew:Celgene: Honoraria. Shamy:Novartis Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees. Elemary:Celgene Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees. Buckstein:Celgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal