Abstract
Background: The majority of MDS patients (pts) have anemia and are treated initially with ESAs. Particularly for lower-risk MDS pts (International Prognostic Scoring System (IPSS) Low and Int-1), once ESAs are no longer effective, treatment options are limited to drugs commonly used for higher-risk MDS, such as hypomethylating agents, or off-label use of immunomodulatory drugs. As a result, most pts receive only transfusion support post-ESA, representing a pt group with an unmet medical need frequently targeted for drug development, for whom long-term outcome is unknown.
Methods: We studied pts diagnosed with lower-risk MDS from 1997-2014 at MDS CRC institutions and treated with ESAs (epoetin alpha (epo) or darbepoetin (darb)). The best response to treatment was categorized per International Working Group 2006 response criteria (hematological improvement (HI), complete response (CR), or partial response (PR)). The primary endpoint was overall survival (OS) at the time of ESA failure, defined as cessation of treatment due to relapse or refractoriness; a secondary endpoint was time to AML transformation or death, from time of response (for responders) or failure (for nonresponders) determination. Descriptive statistics were used for baseline characteristics. The Kaplan Meier method was used to estimate OS and a log rank analysis was used to compare response categories. Cox regression analysis was performed for multivariable analysis.
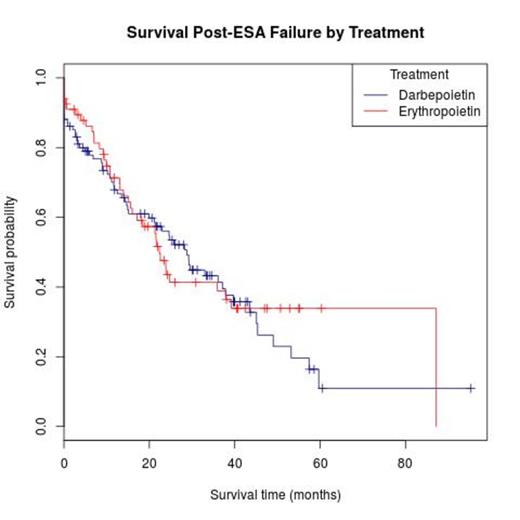
Results: Of 206 patients included in analyses, median age was 71.6 years (range: 25.3-88.1), 36% were female, 5% were African-American, and 11% had t-MDS. WHO categories included RA (14%), RARS (16%), RCMD (42%), MDS-u (6%), del (5q) (4%), RAEB-1 (9%), RAEB-2 (2%), RARS-T (2%), MDS/MPN-u (3%), and CMML-1 (2%), with pts classified as IPSS Low (39%), Int-1 (61%), or IPSS-R Very Low (16%), Low (55%), Intermediate (26%), and High (4%). IPSS cytogenetic risk groups were Good (72%), Intermediate (22%), and Poor (6%). Baseline median hemoglobin was 9.4 g/dl (range: 5.5-14.2), serum epo level was 97.2 (range: 14.2-3899.0), and 11% were transfusion-dependent. Treatment included darb (59%) and epo (41%) at median doses of 300 mcg (range: 100-500) and 40,000 units (range: 5,000-80,000), respectively. Pts remained on therapy for a median of 30.4 weeks (range: 0.0-447.7) and had a median follow-up of 28.4 months (95% confidence interval (CI): 24.5, 45.4). First treatments following ESA failure included azacitidine (41.7%), decitabine (10.2%), lenalidomide (16.6%), experimental drugs (3.1%), other growth factors (13.6%), ATG and/or other immunosuppressants (8%), chemotherapy (0.1%) , transplant (0.1%) and others (6.6%). The overall response rate (ORR) to ESAs was 18.8%, with 0% achieving CR; 0.1% PR; and 18.7% HI. Responses for epo were 17.3% and for darb were 19.8% (p=.67 for difference). For both ESAs, 81.2% of patients had disease refractory to treatment: 69.4% with stable disease and 12% with progressive disease with no significant differences between epo and darb by responder status. Median response duration for epo and darb were 21.9 weeks (range: 3.0 - 447.7) and 39.1 weeks (range: 0.0 - 350.7) respectively (p=0.045). Median survival from the date of diagnosis was 28.4 months (95% CI: 24.5, 45.4), and from ESA failure was 23.9 months (95% CI: 19.9, 33.0): 21.6 months (95% CI: 15.6, 39.2) for epo and 28.8 months (95% CI: 21.2, 39.7) for darb (p=0.99) (Figure). Median time to AML transformation or death was 17.4 Months (95% CI: 14.1, 22.9): 25.4 months for responders and 16.8 months for non-responders (p=.069). For patients who received ESAs for a minimum of 4 months (39% of pts for epo and 61% for darb), ORR was 16.5%, and median survival from ESA failure was 23.0 months (95% CI: 14.7, 33.0): 22.3 months (95% CI: 13.1, NA) for epo and 24.7 months (95% CI: 14.3, 39.7) for darb (p=0.87).
Conclusion: In this large, but uncontrolled cohort, response rates were similar for lower-risk MDS patients treated with epo and darb, though duration was longer for darb. There was a trend for improved outcomes in patients who responded to ESAs. Lower-risk MDS patients treated with ESAs have an OS of less than 2 years from the time of failure, and can thus be considered a high-risk MDS group for whom subsequent therapies are not standardized, representing an unmet medical need.
Sekeres:Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; TetraLogic: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Steensma:Incyte: Consultancy; Amgen: Consultancy; Celgene: Consultancy; Onconova: Consultancy. Komrokji:Incyte: Consultancy, Honoraria, Research Funding; Novartis: Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding; Pharmacyclics: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal