Abstract
Introduction: Approximately 50% of pts with de novoMDS present with cytogenetic abnormalities at diagnosis (Haase D, et al. Ann Hematol. 1995;70:171); deletion (del)5q occurs in ~15% of pts (Haase D, et al. Blood. 2007;110:4385). Cytogenetic abnormalities in addition to del(5q) may be associated with shorter overall survival (OS) and increased risk of progression to acute myeloid leukemia (AML) versus del(5q) alone (Mallo M, et al. Leukemia. 2011;25:110). In 2 large multicenter studies (MDS-003 and MDS-004), lenalidomide (LEN) was evaluated in red blood cell (RBC) transfusion-dependent pts with IPSS Low- or Intermediate-1-risk MDS and del(5q) (List A, et al. N Engl J Med. 2006;355:1456; Fenaux P, et al. Blood. 2011;118:3765). Here, we examine specific cytogenetic abnormalities and outcomes in pts with MDS and del(5q) plus ≥ 2 additional cytogenetic abnormalities from MDS-003 and MDS-004.
Methods: Of 353 pts enrolled, 281 had available cytogenetic data with ≥ 12 evaluable metaphases, and were included. Pts received either LEN 10 mg on days 1-21 of each 28-day cycle, LEN 5 mg or 10 mg continuously, or placebo (PBO). In MDS-004, at week (wk) 16, PBO pts could cross over to LEN 5 mg. Centrally reviewed cytogenetic studies were performed at baseline, and wks 24 and 48 (MDS-003); and at baseline, wks 12 and 24, and every 24 wks thereafter (MDS-004). RBC transfusion independence (TI) ≥ 26 wks, cytogenetic response (CyR), AML progression, OS, and AML-free survival were assessed by baseline cytogenetic complexity in LEN-treated pts with del(5q) plus ≥ 2 additional abnormalities. These patients did not fulfill IPSS lower-risk classification after central pathologic/cytogenetic evaluation.
Results: Of 281 pts, 25 (8.9%) had del(5q) plus ≥ 2 additional abnormalities at baseline. In these pts, the most common additional abnormalities at baseline were -7 (20.0%), del(13q) (20.0%), +21 (16.0%), and del(11q) (16.0%). Baseline characteristics were comparable across the 24 LEN-treated pts with 2 (n = 9), 3 (n = 8), or ≥ 4 (n = 7) additional abnormalities. Rates of RBC-TI ≥ 26 wks were 44.4%, 50.0%, and 28.6% in pts with 2, 3, or ≥ 4 additional abnormalities (P = 0.77), respectively. In pts evaluable for CyR (n = 21), rates of CyR were 33.3%, 28.6%, and 20.0% (P = 1.00), respectively; all cytogenetic responders achieved RBC-TI ≥ 26 wks. The other pts who achieved RBC-TI ≥ 26 wks but did not meet the criteria for CyR showed reductions in the del(5q) clone. No PBO pts achieved CyR; however, 1 pt had a partial response (PR) after crossover to LEN 5 mg. Of the pts randomized to LEN, 4 achieved a complete response (CR) (5 mg, n = 1; 10 mg, n = 3) and 2 achieved a PR (5 mg and 10 mg). Median duration of CyR was 282 days (range 168-957).
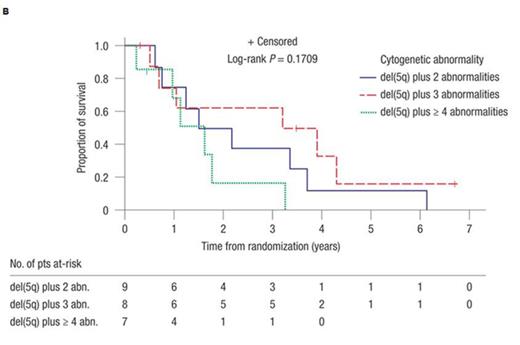
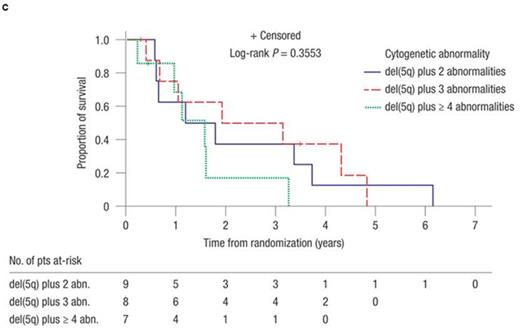
The median number of additional cytogenetic abnormalities in the subset of pts with poor-risk abnormalities (i.e. 17p, 3q, and monosomal abnormalities; n = 7) was 3 versus 2 in pts with good-risk abnormalities (i.e. all other abnormalities; n = 14). Rates of RBC-TI ≥ 26 wks were 28.6% versus 57.1% for the poor-risk versus good-risk groups, respectively. Rates of CyR were 14.3% versus 35.7%, respectively (all CR). In pts with 2, 3, or ≥ 4 additional abnormalities, the 2-year AML progression rates were 56.3% (95% confidence interval [CI] 25.8-89.9), 40.0% (95% CI 14.8-80.5), and 33.3% (95% CI 5.5-94.6), respectively. Median time to AML was 1.8 years (95% CI 0.6-not reached [NR]), 3.1 years (95% CI 0.4-4.8), and NR (95% CI 1.6-NR) (P = 0.75), respectively (Figure 1A). Of 10 pts who developed AML, 6 had involvement of chromosome 7 [del(7q) or -7] at baseline, but presence of -7 did not necessarily portend a poor response in all. Median OS was 1.8 years (95% CI 0.6-3.7), 3.6 years (95% CI 0.5-NR), and 1.6 years (95% CI 0.2-3.3) (P = 0.17) in pts with 2, 3, or ≥ 4 additional abnormalities (Figure 1B). Median AML-free survival was 1.5 years (95% CI 0.6-3.7), 2.5 years (95% CI 0.4-4.8), and 1.6 years (95% CI 0.2-3.3) (P = 0.36), respectively (Figure 1C).
Conclusions: Although RBC-TI and CyR with LEN do occur in pts with del(5q) plus ≥ 2 additional abnormalities, the prognosis is generally dismal and less favorable versus isolated del(5q) and del(5q) plus 1 additional abnormality (Giagounidis A, et al. Blood. 2014;124:abstract 3270). Pts with del(5q) and complex karyotypes are generally associated with IPSS Intermediate-2- or High-risk MDS, which require more intensive treatment approaches, including azacitidine and stem cell transplantation, if feasible.
Giagounidis:Celgene Corporation: Honoraria. List:Celgene Corporation: Honoraria, Research Funding. Hellström-Lindberg:Celgene Corporation: Research Funding. Mufti:Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Morrill:Celgene Corporation: Employment, Equity Ownership. Wu:Celgene Corporation: Employment, Equity Ownership. Skikne:Celgene Corporation: Employment, Equity Ownership. Fenaux:Janssen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Celgene Corporation: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal