Abstract
The second-generation TKIs (2G-TKIs) Dasatinib (DAS) and Nilotinib (NIL) yield faster responses in newly diagnosed chronic phase (CP) CML as compared to Imatinib (IM) however, long-term safety of these agents is a growing concern.
We identified 10 patients with CP CML diagnosed between 08/2013 and 06/2015 who initiated 2G-TKIs and were then switched after optimal response at 3 months to IM. DAS was administered to 8 patients at 100 mg/d and NIL in two patients at 300 mg twice a day. Response to TKI was assessed by quantitative reverse transcriptase polymerase chain reaction (qPCR) for BCR-ABL1. Response to 2G-TKIs after 3 months was as follows: CHR (n=10), 1 log (<10%, n=10), 2 log (<1%, n=7), and 3 log (<0.1%, n=2) reduction of BCR-ABL1 transcripts. Median qPCR at 3 months was 0.77% by IS (range 0-7%).
NIL was discontinued due to grades 2-3 non-hematological toxicities in both patients. DAS was discontinued due to patient or physicians' preference or drug availability. Median time to discontinuation of 2G-TKIs and initiation of IM was 103 days (range, 92 - 120) from diagnosis. IM was started at 400 mg/d and was well tolerated except in 2 patients who required dose-reduction and discontinuation due to grade 2 skin rash (1) and grade 2 anxiety (1). Both patients switched back to DAS at 54 and 228 days after initiation of IM respectively. 9/10 patients that switched to IM were evaluable with a follow up of at least 3 months. These patients have shown a continuous response with 9/9(100%) achieving a 2 log reduction at 6 months. With a median follow-up from initiation of IM of 8 months (range 2-20 months), 5/9 (55.5%) evaluable patients achieved MMR at 6 months and 3/6 MR5 at 9 months from diagnosis.
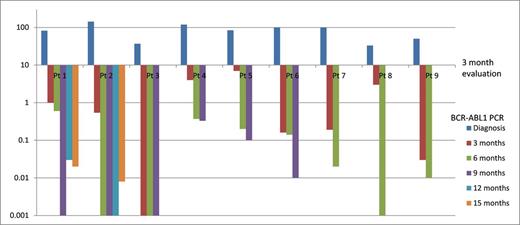
In conclusion, this retrospective analysis shows that IM can be safely and effectively administered following optimal response to 2G-TKIs. Longer follow-up is needed, but with up to 20 months follow-up, all patients showed continuous decrease in their transcripts (Figure 1) and no losses of major molecular response or progression to blast phase were observed. A strategy of induction with 2G-TKIs followed by maintenance with IM is worth evaluating in a prospective trial.
BCR-ABL1 qPCR (IS value) at diagnosis and follow-up after a switch to IM at 3 months from 2G-TKIs.
BCR-ABL1 qPCR (IS value) at diagnosis and follow-up after a switch to IM at 3 months from 2G-TKIs.
Kota:Leukemia Lymphoma Society: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal