Abstract
Background: Chronic, low-grade adverse events (AEs) are common in CML patients (pts) treated with the BCR-ABL1 tyrosine kinase inhibitor (TKI) imatinib (IM) and may decrease quality of life (Efficace, Blood. 2011;118:4554) and adherence to therapy (Marin, J Clin Oncol. 2010;28:2381). These AEs may result in dose modifications and contribute to suboptimal response. Alternative TKIs are available with the potential to reduce AEs, improve tolerability, and support long-term treatment goals. The second-generation TKI dasatinib (DAS) has demonstrated efficacy in pts previously treated with IM in the CA180-034 trial (Shah, Blood. 2014;123:2317). We present the analysis of CA180-400 (NCT01660906), an open-label, multicenter phase 4 study designed to determine if chronic, low-grade nonhematologic AEs in IM-treated pts improve after switching to DAS.
Methods: Adult CML-CP pts who had not failed previous IM therapy (complete hematologic response by 3 mo, partial cytogenetic response [PCyR] by 6 mo, or complete cytogenetic response [CCyR] by 12 mo [Baccarani, Blood. 2013;122:872]) and had a chronic, grade 1/2 nonhematologic IM-related AE persisting for ≥2 mo or recurring ≥3 times in the prior 12 mo, despite best supportive care, were switched to DAS 100 mg once daily until disease progression, treatment failure, unacceptable AE, withdrawal of consent, or study discontinuation at 12 mo. The primary endpoint was the frequency of chronic, grade 1/2 nonhematologic IM-related AEs that decreased in grade or resolved within 3 mo following DAS initiation. Treatment-emergent DAS-related AEs and pt-reported symptom burden using MD Anderson Symptom Inventory (MDASI)-CML symptom severity and interference scores (0-10 scale; 10=worst) were also evaluated 3 mo following the switch to DAS.
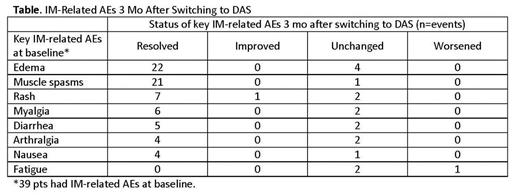
Results: Thirty-nine pts were enrolled in the trial. Median age was 57 y (range 23-81 y), with 31% ≥65 y. Median duration of prior IM was 47 mo and 49% had received <400 mg IM. Best response to IM at study entry was PCyR in 5% and CCyR in 69%; 69% also had major molecular response. A total of 123 chronic, grade 1/2 IM-related AEs were reported at baseline in the 39 pts; muscle spasms, facial edema, rash, myalgia, diarrhea, arthralgia, periorbital edema, and nausea each occurred in ≥10% of pts. Of the 123 AEs, 91 (74%) resolved completely, 2 (2%) improved in grade, 29 (24%) remained unchanged, and 1 (1%) worsened in grade within 3 mo of switching to DAS. All instances of edema either resolved or did not change, and similar results were reported for muscle spasms, rash, myalgia, diarrhea, arthralgia, and nausea (Table). Fatigue was the only IM-related AE that worsened on DAS. The majority of pts experienced improvement in ≥1 IM-related AEs, without the worsening of any other IM-related AE (overall, 34/39 [87%]; <65 y, 24/27 [89%]; ≥65 y, 10/12 [83%]). Treatment-emergent DAS-related nonhematologic AEs occurred in 29 (74%) pts within 3 mo and were mostly grade 1/2 (grade 3/4, 8/39 [21%]), including headache in 12, fatigue in 9, diarrhea in 7, and pleural effusion in 3. No pts had pulmonary arterial hypertension 3 mo following the switch to DAS. Two pts discontinued DAS due to toxicity (pleural effusion, extremity pain; sinus tachycardia, nausea, fatigue, headache). MDASI-CML symptom severity scores changed -0.6, -1.1, -1.4, -1.4 from baseline to 2 wk, 4 wk, 8 wk and 3 mo, respectively, and symptom interference scores changed -0.5, -1.0, -1.1, and -1.2 from baseline to 2 wk, 4 wk, 8 wk, and 3 mo, respectively, following DAS initiation, suggesting that symptom burden was reduced following the switch to DAS.
Conclusions: In CA180-400, the majority of chronic, low-grade nonhematologic IM-related AEs resolved, and most others improved after switching to DAS. Pt-reported symptom burden was lower on DAS, and most physician-reported AEs were low-grade. Final, 1-year follow-up data, including efficacy, will be presented for all enrolled pts.
Cleeland:Amgen Inc.: Consultancy. Saussele:Pfizer: Honoraria, Other: Travel grant; Novartis Pharma: Honoraria, Other: Travel grant, Research Funding; ARIAD: Honoraria; BMS: Honoraria, Other: Travel grant, Research Funding. Williams:Amgen: Consultancy; Novartis: Research Funding. Mohamed:BMS: Employment, Equity Ownership. Pinilla-Ibarz:Teva: Consultancy, Speakers Bureau; ARIAD Pharmaceuticals, Inc.: Consultancy, Other: Consulting & Advisory Role, Research Funding; Pfizer: Consultancy, Other: Consulting & Advisory Role, Research Funding, Speakers Bureau; Novartis: Consultancy, Other: Consulting & Advisory Role, Research Funding; BMS: Consultancy, Honoraria, Other: Consulting & Advisory Role, Speakers Bureau. Abruzzese:BMS, Novartis, Pfizer, Ariad: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal