Abstract
Introduction
MCL is an incurable aggressive B-cell lymphoma with a poor overall prognosis. For patients with MCL who fail initial therapy (ie, with relapsed or refractory [R/R] disease), treatment options historically have been limited. While remission duration is generally short, the goal of therapy has been to achieve remission while balancing treatment-related toxicities. Consequently, a substantial proportion of patients continuously suffer from lymphoma symptoms and other disease signs, such as itching and trouble sleeping or concentrating. Additionally, worries and high emotional sensitivity lead to reduced functional status and well-being. Therefore, it is crucial to any treatment to maintain and improve the functional status and well-being of patients with R/R MCL for as long as possible throughout the treatment period.
Methods
RAY (MCL3001) is a phase 3, randomized, open-label, multicenter study in patients with R/R MCL. Patients were randomized in a 1:1 ratio to receive ibrutinib (560 mg once daily) or temsirolimus (175 mg on days 1, 8, and 15 of Cycle 1; 75 mg on days 1, 8, and 15 of subsequent cycles). Stratification factors were the number of prior lines of therapy (1-2 vs ³3) and simplified MCL international prognostic index risk (low [0-3] vs intermediate [4-5] vs high [6-11]). Patients in both arms received treatment until disease progression or unacceptable toxicity. Primary end point of the study was progression-free survival (PFS), as assessed by an independent review committee. Lymphoma symptoms were assessed using the Functional Assessment of Cancer Therapy-Lymphoma (FACT-Lym) questionnaire lymphoma subscale. The FACT-Lym was administered before any tests, procedures, or other consultations, and was used until disease progression, death, or the clinical cutoff, whichever came first. Median time to worsening (TTW), a prespecified secondary end point, and time to clinically meaningful improvement (TTI) on the lymphoma subscale were estimated. Worsening was defined as a ≥ 5-point decrease from baseline and clinically meaningful improvement as a ≥ 5-point increase from baseline. TTW and TTI on the lymphoma subscale were examined using Kaplan-Meier methods.
Results
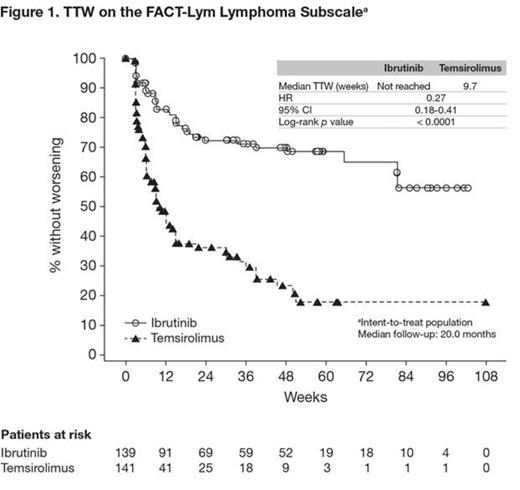
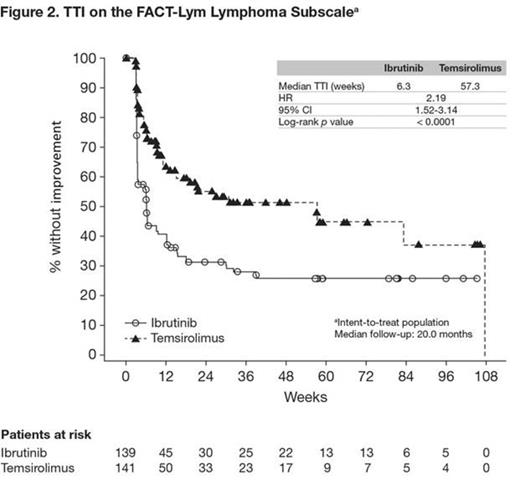
In total, 280 patients were randomized to ibrutinib (n = 139) or temsirolimus (n = 141); 253 patients (ibrutinib, n = 130; temsirolimus, n = 123) provided FACT-Lym lymphoma subscale responses at baseline. Patients were compliant to therapy, and attrition was balanced between treatment arms. Disease characteristics and demographics at baseline (including FACT-Lym lymphoma subscale scores) were also generally well balanced. The study met its primary end point with a statistically significant 57% reduction in disease progression or death (PFS) with ibrutinib versus temsirolimus (hazard ratio [HR], 0.43; 95% confidence interval [CI], 0.32-0.58; p < 0.0001). The median PFS was 14.6 months for the ibrutinib arm and 6.2 months for the temsirolimus arm. When adjusted for exposure, incidence of TEAEs was lower for the ibrutinib arm than the temsirolimus arm. The proportion of patients with worsening from baseline on the FACT-Lym lymphoma subscale was lower for ibrutinib compared with temsirolimus (26.6% vs 51.8%, respectively). Median TTW was not reached with ibrutinib versus 9.7 weeks with temsirolimus, with a statistically significant reduction in the risk of worsening of 73% (p < 0.0001; Figure 1). The proportion of patients with a clinically meaningful improvement from baseline on the FACT-Lym lymphoma subscale was higher for ibrutinib compared with temsirolimus (61.9% vs 35.5%, respectively). Median TTI was shorter for ibrutinib compared with temsirolimus (6.3 weeks vs 57.3 weeks, respectively; HR, 2.19; p < 0.0001; Figure 2).
Conclusions
RAY (MCL3001) is the first trial comparing different targeted approaches in R/R MCL. The presented data indicate that ibrutinib is associated with greater improvements and less worsening in lymphoma symptoms compared with temsirolimus, as measured by the lymphoma subscale of the FACT-Lym. These results suggest that the superior efficacy results and favorable safety profile of ibrutinib are accompanied by better lymphoma symptom outcomes, indicating significant clinical benefit for the majority of patients with R/R MCL.
Hess:Pfizer, Janssen, Roche, Mundipharma: Honoraria, Research Funding; Janssen, Roche, Celgene, Novartis: Consultancy. Rule:J&J: Consultancy, Other: Travel reimbursement, Research Funding; Roche: Consultancy, Other: Travel reimbursement; Celgene: Consultancy, Other: Travel reimbursement; Gilead: Research Funding. Jurczak:CELLTRION, Inc,: Research Funding. Rusconi:Roche: Honoraria. Witzens-Harig:Pfizer: Honoraria, Research Funding; Roche: Honoraria. Bandyopadhyay:Janssen: Employment. Goldberg:Janssen: Employment. Bothos:Janssen: Employment. Enny:Janssen: Employment. Vermeulen:Janssen: Employment. Traina:Janssen: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal