Abstract
Introduction: While immunosuppression for solid organ transplant is associated with an increased risk of lymphoproliferative disease (LPD), this has been more difficult to establish in autoimmune disorders, even though patients are often treated with similar agents. One reason is that autoimmune disease may elevate baseline LPD risk. However, associations have been shown with certain rare types of LPD; most strikingly hepatosplenic lymphoma, now known to occur as a consequence of anti-TNF-alpha therapy in young men with inflammatory bowel disease (IBD) (J Ped Gast Nutr. 2007; 44:265-7).
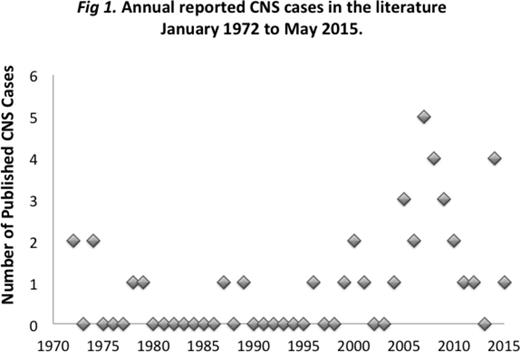
We have noticed a rise in the incidence of another rare lymphoma in autoimmune disease patients: primary central nervous system (PCNS) LPD. Six cases have been diagnosed at our institution since 2010, with none before that dating back to onset of electronic records in 1986. All of these patients were taking mycophenolate mofetil (MMF) and/or thiopurines. A similar rise in reported cases has been seen in the literature (Fig 1) with suggestion of but no direct association with drug treatment shown. We systematically investigated this trend.
Methods: We searched our pathology database to identify all LPD cases diagnosed over a 28-year period in patients treated for autoimmune disease as well as all similar cases involving the CNS reported in the literature over the past 40 years. Statistical analyses were performed using the Fisher's exact test.
Results: We identified 44 cases of LPD arising in patients treated for autoimmune disease, including 6 with PCNS disease (Table 1). Of LPDs in patients on anti-TNF-alpha agents, 4/5 had a T-cell phenotype, and 3 had IBD. By contrast, in patients who developed LPD while taking methotrexate, the majority for rheumatoid arthritis, only 1/18 had a T-cell phenotype. Instead they were categorized as polymorphous, Hodgkin or large B-cell morphologies, which were frequently EBV-positive (67%), but never involved the brain (0/18). The LPDs arising in patients on MMF and/or thiopurines showed a similar morphologic profile but were more likely to involve the CNS. In particular, MMF was significantly associated with PCNS compared to non-CNS disease (p<0.001), and the only patient on MMF that developed an LPD outside the CNS was taking MMF in combination with cyclosporine. The most common underlying disorders in PCNS disease were myasthenia gravis (MG) and IBD.
We reviewed all cases of CNS LPD in patients treated for autoimmune disease reported in the literature (34 reports, 40 patients), including 32 with PCNS LPD and 8 with secondary CNS involvement. The vast majority of PCNS cases arose in patients taking MMF and/or thiopurines (29/32), but only MMF was significantly associated with primary compared to secondary CNS involvement (p<0.05). The most common underlying disease in PCNS patients was systemic lupus erythematosus (SLE) (10/32), followed by IBD and MG. No patients with secondary CNS involvement had SLE.
Conclusions: While the overall risk of LPD in the context of autoimmune disease treatment has been controversial, the interaction between drug type and individual patient characteristics may dramatically increase risk for certain lymphomas. We now demonstrate a significant association between use of MMF and PCNS LPD, which appears to cluster in patients with a history of SLE, MG or IBD. Of interest, all 3 autoimmune patients in the JHH database who developed PCNS LPD following solid organ transplant (not shown) also had SLE. While methotrexate never produced a PCNS LPD in our series, it has been infrequently found in the literature.
There is no evidence of an increased baseline risk of PCNS LPD in autoimmune patients; indeed, only one reported case in an untreated patient could be identified in the literature (J Rheum 1978; 5:75-78). In addition, EBV-associated PCNS lymphoma is virtually always seen in the context of immunosuppression. Further investigation into the increased risk of specific types of LPD with immunosuppressive treatment is warranted with significant implications for tailoring treatment options.
Demographics of JHH and Reported CNS Cases ('*' p<0.05).
| . | JHH . | All Reported CNS Cases (Literature) . | ||
|---|---|---|---|---|
| PCNS | Non-CNS | PCNS | 2o CNS | |
| No. Cases | 6 | 38 | 32 | 8 |
| Age (range) | 69(27-77) | 61(18-77) | 57(27-88) | 62(15-71) |
| % Male | 50% | 50% | 32% | 25% |
| Deceased | 17% | 37% | 45% | 40% |
| EBV | 100% | 69% | 95% | 100% |
| MMF | 80%* | 3% | 41%* | 0 |
| Thiopurines | 40% | 23% | 72% | 75% |
| Methotrexate | 0 | 58% | 13% | 38% |
| SLE | 0 | 9% | 31%* | 0 |
| MG | 33% | 3% | 19% | 0 |
| IBD | 33% | 24% | 19% | 38% |
| . | JHH . | All Reported CNS Cases (Literature) . | ||
|---|---|---|---|---|
| PCNS | Non-CNS | PCNS | 2o CNS | |
| No. Cases | 6 | 38 | 32 | 8 |
| Age (range) | 69(27-77) | 61(18-77) | 57(27-88) | 62(15-71) |
| % Male | 50% | 50% | 32% | 25% |
| Deceased | 17% | 37% | 45% | 40% |
| EBV | 100% | 69% | 95% | 100% |
| MMF | 80%* | 3% | 41%* | 0 |
| Thiopurines | 40% | 23% | 72% | 75% |
| Methotrexate | 0 | 58% | 13% | 38% |
| SLE | 0 | 9% | 31%* | 0 |
| MG | 33% | 3% | 19% | 0 |
| IBD | 33% | 24% | 19% | 38% |
Borowitz:Becton Dickinson Biosciences, Medimmune: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal