Abstract
Background
18 F-FDG PET is currently used in diffuse large B-cell lymphoma (DLBCL) for staging and evaluation of therapeutic efficacy at various time points. Nevertheless, the predictive value of interim PET/CT (iPET/CT) has not been consistent throughout the studies. Since FDG is not a tumor-specific substance, it may accumulate to the point of being detected in a variety of benign conditions or infectious lesions, which may give rise to false positive interpretation. Particularly, iPET/CT assessment in patients with multifocal, non-contiguous involvement at extranodal (EN) sites may result in a false determination of prognosis due to tracer uptake of inflammatory or physiologic anatomic sites, which could contribute to the variability in outcomes and the poor reproducibility. Therefore, the purpose of this study is to investigate the predictive accuracy of iPET/CT response based on visual and quantitative SUV-based assessments in patients with DLBCL and EN involvements.
Methods
iPET/CT responses for 163 patients with newly diagnosed DLBCL and EN involvements were investigated retrospectively. iPET/CT responses were based on the visual and quantitative SUV-based assessments. Briefly, the assessment of PET/CT was performed at the time of diagnosis, at the third or fourth cycles and at the completion of R-CHOP. For visual assessment, the five-point scale (5-PS) based on the Deauville criteria was used and graded as negative or positive by comparison with initial PET/CT scan and grade 1-3 were considered as negative and grade 4-5 were considered the residual metabolic response. Second, we classified patients using the quantitative analysis of 18 F-FDG uptake changes based on the percentage of SUVmax reduction (DSUVmax) between initial and interim PET/CT scans. The cutoff points of DSUVmax were 65.7% based on previous reports.
Results
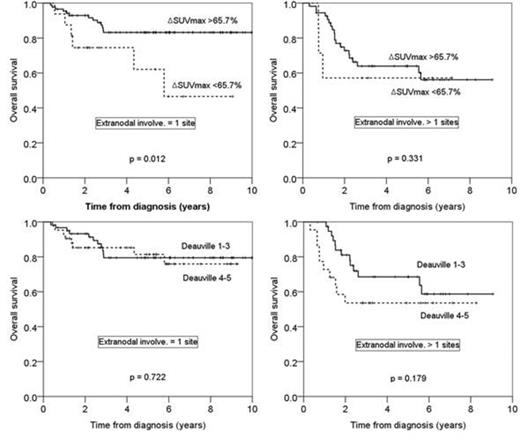
Median age was 61 years (range 18-83 years) and 88 patients (54.0%) in advanced disease (III/IV). Patients were classified according to the IPI risk with 95 patients (58.3%) being classified as low or low-intermediate and 68 patients (41.7%) as high-intermediate or high risk. Number of extranodal site(s) were 1 site in 102 patients (62.6%), 2 sites in 39 (23.9), 3 sites in 18 (11.0), and 4 sites in 4 (2.5%). iPET/CT responses based on visual analysis were classified into grade 1-3 of 5-PS in 99 patients (60.7%) and grade 4-5 in 64 (39.3%), and based on SUV-based, classified into higher the cutoff of DSUVmax (>65.7%) in 140 patients (85.9%) and lower (<65.7%) in 23 patients (14.1%), respectively. On visual assessment, iPET/CT-positive patients had no difference of relapse rates (28.3±5.4%) compared to those of iPET/CT-negative patients (23.1±4.5%) (p=0.419). Among the patients with 5-PS grade 4-5, 46 patients (71.9%) achieved higher the optimal cutoff of DSUVmax (>67.5%).
The 5-year overall survival (OS) rates and progression free survival (PFS) rates were 75.6±3.8% vs 60.6±11.7% (p=0.056), and 77.9±3.7% vs 55.9±12.1% (p=0.007) depending on the cutoff of DSUVmax, respectively. Among the patients with 1 EN involvement, DSUVmax successfully predict the long-term outcomes in terms of 5yr-OS (83.2±4.3% vs 62.1±14.6%, p=0.012) and PFS (86.9±3.9% vs 41.3±16.6%, p<0.001), while for those with more than 1 EN involvements, DSUVmax failed to predict long-term outcomes. In the multivariate analysis, DSUVmax <65.7% (HR=2.675, 95% CI 1.304-5.486, p=0.007), ECOG 2-3 (HR=2.553, 95% CI 1.228-5.309, p=0.012), and the involvement of more than 1 EN sites (HR=2.370, 95% CI 1.247-4.504, p=0.008) were unfavorable factors for PFS. Visual assessments based on Deauville 5-PS score could not predict the disease progression or long-term outcomes regardless of the number of extranodal involvements or IPI risk groups.
Conclusion
The quantitative SUV-based assessments in iPET/CT could have significant potential as a prognostic predictor of PFS and OS, especially in the patients with 1 EN site involvement. However, the visual assessments have the limitations to predict long-term outcomes with high false positive rates at EN involvements.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal