Abstract
Classic Hodgkin lymphoma (cHL) is characterized by a rich non-malignant immune infiltrate. T-cells are key components of the antitumoral immune response and studies characterizing subsets in cHL have yielded conflicting results. Initial studies suggested a predominance of TH2-polarized CD4+ T-cells, thought to allow tumor progression due to exhaustion and hypofunctionality. More recent data contest these findings, supporting the theory of tumor progression through evasion from a TH1-rich infiltrate that is potentially functional.
The role of tumor evasion in cHL has been highlighted by compelling early clinical data with the use of PD-1 blockade in patients with advanced disease. A similar trial in patients with non-Hodgkin lymphoma (NHL) yielded far more modest results. Intrinsic differences in T-cell subpopulations in the tumor microenvironment may correlate to response to immune checkpoint inhibitor therapy.
CyTOF or mass cytometry is a platform able to evaluate more than 45 simultaneous parameters on a single-cell level using nonradioactive nonbiological isotopes tagged to monoclonal antibodies. Measurements are made based on mass spectrometry, avoiding the hurdles of interference and spectral overlap experienced with fluorochromes. This constitutes an ideal tool for the study of the tumor microenvironment given its ability to assess a large number of parameters and resolve differences in a heterogeneous population.
We hypothesize that the phenotype of intratumoral lymphocytes in cHL identifies T-cells that can effectively eradicate malignant cells. To test this hypothesis, we compared the phenotype of intratumoral T-cells in cHL to that of NHL and nodular lymphocyte-predominant Hodgkin Lymphoma (nlpHL). Tonsil and hyperplastic lymph node (LN) tissues were used as normal controls.
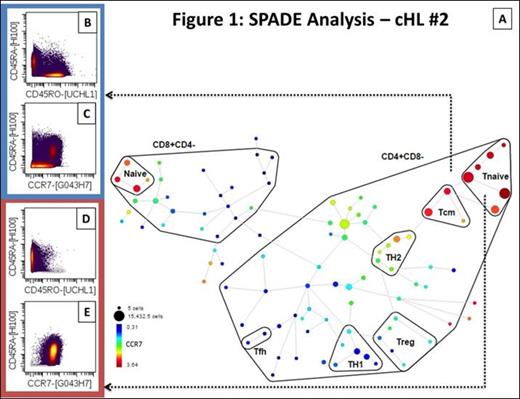
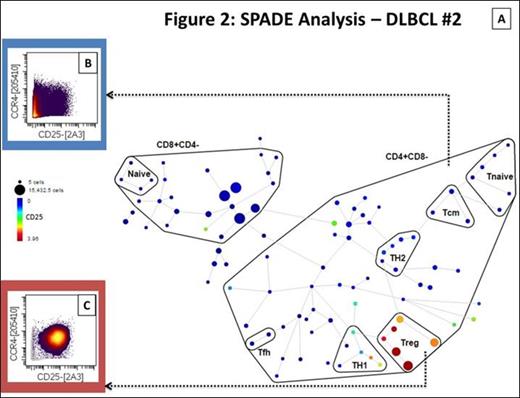
Single-cell suspensions created from tumor specimens were stained with a metal-tagged antibody panel containing 31 surface markers and acquired on CyTOF. Multiparametric data analysis was performed on Cytobank using spanning-tree progression analysis of density-normalized events (SPADE) and t-Distributed Stochastic Neighbor Embedding (viSNE) algorithms. Inferential statistical analyses were performed with JMP®, Version 10.0.0 (SAS Institute Inc., Cary, NC, 1989-2007) using two-tailed tests and a 95% confidence interval. Cell subsets are expressed as percentages of parent population (CD45+CD3+CD19-).
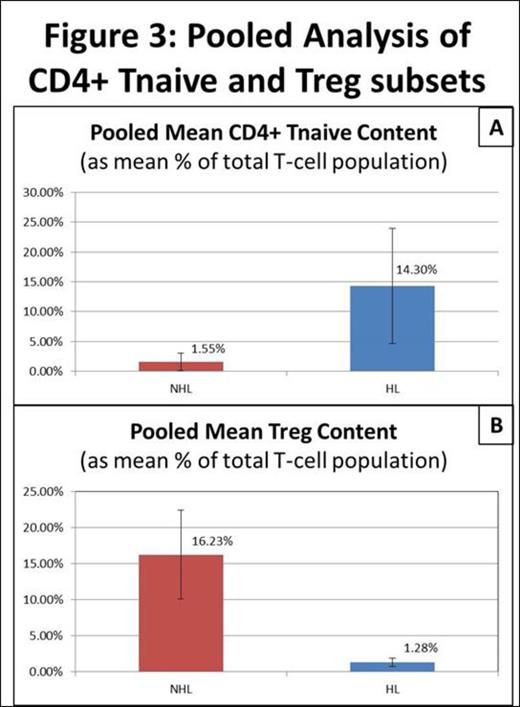
A total of 10 samples were studied (4 cHL, 1 nlpHL, 3 NHL, 1 tonsil, 1 LN). The total T-cell population ranged from 30.52 to 67.05% in cHL and 15.36 to 47% in NHL compared to 4.02% and 24.58% in tonsil and LN respectively. The CD4+ T-cell subset ranged from 58.05 to 35.3% in cHL, 50.03 to 82.61% in NHL and corresponded to 82.74% and 87.07% in tonsil and LN respectively. SPADE analysis identified two areas of asymmetric frequency of events amongst samples (figure 1 and 2). The CD4+ Tnaive subset (CD4+CD45RA+CCR7+) ranged from 7.8 to 31.2% of total T-cells in cHL compared to 10.7% in nlpHL, 0.17 to 3.02% in NHL and 6.2 to 6.7% in controls. The pooled mean frequency of CD4+ Tnaive subset was significantly higher in HL (cHL + nlpHL) compared to NHL (14.3% vs. 1.55%; p<0.05; figure 3A). The regulatory T-cell subset (Treg; CD25+CCR4+) ranged from 0.49 to 1.84% of total T-cells in HL compared to 9.3 to 21.04% in NHL, and 4.45 to 8.28% in controls. The pooled mean frequency of the Treg subset was significantly smaller in HL compared to NHL (1.28% vs. 16.23%; p<0.05; figure 3B).
Our data supports the use of mass cytometry as a platform to study the tumor microenvironment in B-cell lymphomas. Multiparametric data analysis revealed significant differences in the intratumoral T-cell population between HL and NHL samples, namely in the CD4+ Tnaive and Treg subsets. Further validation in a larger sample is underway and will include panels to evaluate intracellular cytokine production and cell signaling pathways. Correlation between specific intratumoral T-cell phenotypic signatures and clinical outcomes may identify prognostic and predictive characteristics and provide insight to mechanisms of resistance to immunotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal