The MLL--AF9 fusion oncogene is associated with acute leukemias in adults and children. Retroviral expression of MLL-AF9 in mouse hematopoietic stem/progenitor cells (HSPCs) rapidly induces acute myeloid leukemia (AML) and therefore serves as an excellent model to study MLL-AF9 induced leukemogenesis. We previously showed that miR-29 is highly expressed in human AML and positively regulates hematopoietic stem cell (HSC) self-renewal. Consistent with a role in AML, higher expression of miR-29 predicts poor survival in AML patients using the TCGA dataset. In addition, transduction of MLL-AF9 into microRNA-29 (miR-29a/b1) null Lin-c-Kit+Sca1+ (LSK) cells was sufficient to induce leukemic colony formation in vitro similar to WT LSK cells, but transplantation of MLL-AF9+ miR-29 null cells into lethally irradiated recipients resulted in an increased disease latency (median - 56 vs 151 days, p<0.001). In addition, with a short time transduction (1 weeks) of MLL-AF9, mice of miR-29 null recipients exhibited long-term donor-derived lymphomyeloid engraftment. MiR-29 null and MLL-AF9 positive HSPCs were serially transplantable, and did not generate AML during a 2 year time period during with 5 times transplantation, suggesting no significant alteration in HSPC self-renewal.
To understand why miR-29 null cells are relative resistant to MLL-AF9 mediated transformation, we first determined whether miR-29 null cells induce genes properly following MLL-AF9 transduction. RNA-sequencing of miR-29 null spleen B cells revealed that 1147 and 559 genes were significantly up- or down-regulated (adjusted p < 0.001) following MLL-AF9 transduction. The absence of miR-29 resulted in dysregulated expression of class I Hox genes, which were nearly undetectable in miR-29 null mature B cells; however, these genes were significantly up-regulated in MLL-AF9+ cells, including Meis1 (2.68-fold), HoxA5 (4.85-fold), Hoxa7 (3.46-fold), HoxA3 (2.43-fold), and HoxA6 (3.35-fold). One important exception was HoxA9, which was undetectable in both MLL-AF9+ or MLL-AF9- B cells, indicating the inability of MLL-AF9 to induce one of its key leukemogenic mediators, HoxA9, in the absence of miR-29. These data suggest the inability of MLL-AF9 to induce AML in the miR-29 null context might due to its inability to properly induce sufficient MLL-AF9 targets. Analysis of dysregulated genes reveals that the top pathways positively regulated by MLL-AF9 were cytokine receptor interactions, and adhesion molecules.
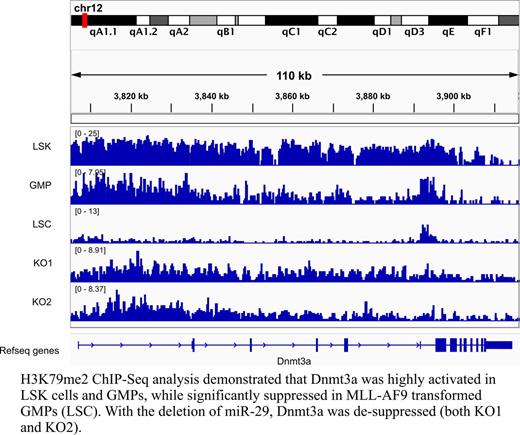
We found Meis1, Ccna2, Hoxa5 and Hoxa9 were significantly down-regulated in miR-29 null versus WT LSK cells, but were similarly induced in MLL-AF9 transformed blasts, suggesting that increased AML latency was not due to lack of ability to induce these known key regulators of leukemogenesis. As miR-29 directly binds to the 3'-UTR of Dnmt3a, we sought to determine whether epigenetic dysregulation underlies the leukemic phenotype in miR-29 null cells. Performing ChIP-Seq for the active epigenetic mark H3K79me2 in c-Kit+Mac-1+ blasts, we identified 4281 and 3649 genes with this mark from WT and miR-29 null blasts, respectively, with an overlap of 3164 genes (66.39%). Next, we compared our data to H3K79me2 positive genes from WT LSK cells, granulocyte-macrophage progenitors (GMP), and MLL-AF9 transformed leukemic-GMP (L-GMP) and found that 45.6%, 48.0%, and 47.1% of these genes were also identified in MLL-AF9+miR-29 null cells, indicating that miR-29 loss reduces the induction ability of MLL-AF9 targets. We also observed that 379 genes associated with the H3K79me2 mark in both normal LSK cells and MLL-AF9+miR-29 null blasts were absent in L-GMP, suggesting that these genes confer self-renewal and proliferation capacity to normal HSCs, that their suppression of these genes are important in MLL-AF9 induction of AML, and that their reactivation in miR-29 null cells compromises MLL-AF9 leukemogenic ability. Interestingly, we were able to identify several potential miR-29 targets including Akt3, Map4k4, and Dnmt3a.
Overall, our studies demonstrate that deletion of miR-29 abrogates the leukemogenesis capacity of MLL-AF9, and miR-29 null mice appear to be an important tool for identifying critical mediators of MLL-AF9 induced AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal