Abstract
Introduction: Immune Thrombocytopenia (ITP) is still diagnosed by exclusion of many other causes for thrombocytopenia. In order to prevent misdiagnosis, an ITP-specific diagnostic test would be very helpful. In addition, characterization of glycoprotein specificity of platelet autoantibodies may explain (the severity of) bleeding symptoms and response to therapy. In this regard, we optimized the cut-off value of the direct monoclonal antibody immobilization of platelet antigens (MAIPA) assay for detection of platelet glycoprotein directed autoantibodies and re-evaluated its sensitivity and specificity for the diagnosis of ITP.
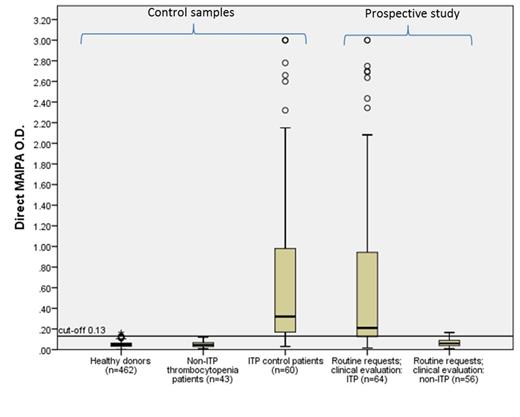
Materials and Methods: The MAIPA was performed as part of our routine protocol, described by Kiefel et al. (1985). For the determination of a new cut-off value, and to calculate the sensitivity and specificity, blood samples were tested from 462 healthy blood donors and 43 non-immune-mediated thrombocytopenic patients, suffering from either hematological malignancies or aplastic anemia (n=20), hepato-splenomegalic pooling (n=3), drug-induced thrombocytopenias (n=4), viral infections (n=6), pregnancy related thrombocytopenia (n=7), pseudothrombocytopenias (n=2) and microangiopathy (n=1) and from 60 known ITP patients. We then have tested 120 prospectively collected samples from thrombocytopenic patients, sent for diagnostic tests to our laboratory, and categorized these samples based on subsequently obtained clinical evaluation into 'most likely ITP' (n=64) or 'most likely non-ITP' (n=56).
Results: The calculated direct MAIPA sensitivity and specificity, using a cut-off value of E=0.130, in the ITP and non-ITP control groups (n=103) were 85% (95% CI, 73-93%) and 100% (95% CI, 92-100%), respectively (see Figure). The platelet auto-antibodies in the ITP control group (n=60) were directed against glycoprotein (GP)IIb/IIIa (66.7%), GPIb/IX (60%), GPV (51.7%), GPIa/IIa (40.6%) and/or GPIV (26.9%). The calculated sensitivity and specificity for detection of platelet auto-antibodies in the prospective diagnosed ITP and non-ITP patient control groups (n=120) were 75% (95% CI, 63-85%) and 96% (95% CI, 88-100%), respectively (see Figure). For this group of patients, the direct MAIPA showed, for diagnosis of ITP, a negative predictive value (NPV) of 77% (95% CI, 66-86%) and a positive predictive value (PPV) of 96% (95% CI, 86-100%).
Furthermore, in 23 ITP patients the sequential sampling in a rituximab-treatment protocol showed platelet counts that were significantly and inversely correlated with the direct-MAIPA extinctions (p=0.006). In this respect, we excluded that higher platelet counts impaired the detection of platelet autoantibodies - e.g. by diluting them over an higher platelet mass-since autoantibodies were successfully detected in samples from ITP patients (n=4) with, as a result of splenectomy, platelet counts above 100 x 109/L and in untreated ITP patients with platelet counts between 75 and 100 x 109/L. These findings may implicate that response to rituximab as reflected by a rise in platelet counts is dependent on antibody presence, but the mechanism of effective lowering of platelet autoantibody levels by rituximab is still unclear.
In conclusion, the revisited direct MAIPA showed to be a valuable technique for the detection of platelet autoantibodies both at diagnosis and during treatment and can possibly become a guide for optimizing therapy towards a more personalized treatment of ITP.
Direct MAIPA O.D. above 0.13 is considered positive. Control samples: historically well characterized ITP patients. Prospective study: requests for serological ITP diagnostics, after final clinical evaluation classified as ITP or non-ITP.
Schipperus:Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal