Abstract
Introduction
Despite current ABO/RhD matching and strict antibody screening policies, still every year transfused patients experience life-threatening hemolytic reactions due to boostering of previously immunization to red blood cell (RBC) antigens. Prevention can be optimized by administering RBC units at least matched on the most immunogenic antigens to high risk patients. In this respect, we set out to assess the immunogenicity of RBC antigens.
Methods
We performed an incident new user cohort study among previously non-transfused, non-alloimmunized patients who received RBC transfusions between 2006 and 2013 in six Dutch hospitals. Patients developing alloantibodies were followed up until their first RBC alloantibody identification and all non-alloimmunized patients until the last negative screen. To compute dose-specific alloimmunization risks and thereby evaluate the immunogenicity of various RBC antigens, only antigen-positive units transfused to all patients lacking this antigen should be considered. Per definition, alloimmunized patients met this condition. RBC phenotypes of non-alloimmunized patients were however unknown since phenotyping is routinely limited to ABO and RhD antigens. For each RBC antigen we therefore randomly extracted a subgroup of non-immunized patients whose size was based on the known proportion of antigen-negative individuals in the Caucasian population. The given antigen-positive units transfused to these 'antigen-negative cohorts' functioned to estimate the number of antigen-positive units transfused to the true antigen-negative, non-alloimmunized individuals in the source population. Multiple imputation was used to complete the dataset regarding some missing donor antigen phenotypes. We then calculated cumulative immunization incidences for each RBC antigen according to the total number of mismatched (i.e. antigen-positive) units using Kaplan-Meier survival tables.
Women under 45 years of age were analyzed separately as in the Netherlands they receive c, E and K matched blood.
Results
In 474 of 21,512 patients (2.0%), 537 first formed antibodies were detected, the majority against E and K antigens. Cumulative immunization incidences after 40 RBC units transfused increased to 7.6% (CI 4.8-11.2). Due to lower frequencies of Rh and K immunizations, women under 45 years, who received blood matched on these antigens, demonstrated significantly lower cumulative immunization incidences compared to the remainder of the study population (4.4% (CI 0.2-20.5) versus 7.6% (CI 4.8-11.2) after 40 units received, log-rank p 0.013).
Anti-c was only formed by RhD-positive patients while the lack of RhD expression led to significantly less E immunizations (cumulative immunization incidences 1.7% (CI 0.0-32.0) and 3.7% (CI 1.4-7.9) after 40 RBC units received for RhD-negative and RhD-positive patients respectively (log-rank p<0.001). RhD phenotype did not determine the risk of immunization against other RBC antigens.
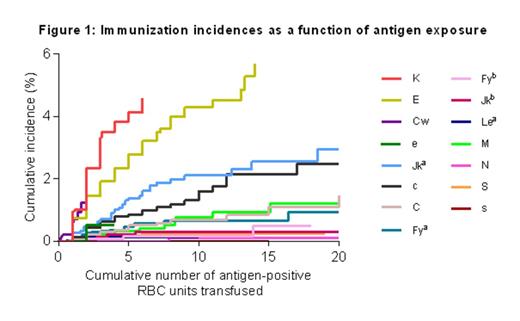
K, E and Cw were the most immunogenic antigens (cumulative immunization incidences 2.4% (CI 1.0-4.8), 1.5% (CI 0.6-3.0) and 1.2% (CI 0.0-10.8) respectively after 2 antigen-positive units, figure 1). These antigens were 8.8, 5.5 and 4.5 times as immunogenic as Fya. The order of antigen immunogenicity was followed by e, Jka and c with rates of 1.9, 1.9 and 1.6 compared to Fya (figure 2).
Conclusion
The risk of alloimmunization is related to antigen exposure and thereby to antigen frequency and total RBC exposure. Reducing antigen exposure by RhD matching protects RhD-negative patients against E immunization due to strong RHD and RHcE gene linkage. Most importantly, in this so far largest database, we determined the immunogenic order of RBC antigens and quantified their dose-corrected immunization risks with K and E being the most immunogenic followed by Cw, e, Jka and c. In this regard and as anti-Jka can induce serious transfusion reactions, we recommend to add Jka matching to current Rh and K matching strategies in high risk patients and whenever possible.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal