Abstract
Background: Patients with newly diagnosed acute lymphoblastic leukemia (ALL) are known to be hypercoagulable due to their disease and from side effects of chemotherapy. Studies have shown rates of venous thromboembolism (VTE) between 5-70% in patients with ALL (depending on various definitions and modalities of investigation) (Caruso et al, 2006, Mitchell et al, 2003). Established methods to monitor hypercoagulability or predict VTE do not exist. We have previously described methods using thromboelastography (TEG) to detect hypercoagulability (Ko et al., 2013). We believe that a global assay of hemostasis such as TEG holds promise to monitor hypercoagulability in patients with newly diagnosed ALL.
Methods: This was a prospective study of newly diagnosed ALL patients between 1-21 years in whom a central venous catheter (either a peripherally inserted central catheter [PICC] or a subcutaneously implanted port) was placed at diagnosis. Patients were enrolled at Children's Hospital Los Angeles from September 2012 until July 2015. None of the patients had a known past medical or family history of thrombosis or bleeding or clotting disorders and none were taking any medications (other than induction chemotherapy) that could affect coagulation (e.g. NSAIDs). Patients had complete blood counts (CBC), TEG, and markers of thrombin generation (quantitative D-dimers, thrombin:antithrombin complexes, and prothrombin fragment 1.2) performed before initiation of treatment and then once weekly during induction until they either presented with a symptomatic VTE or completed Induction therapy. All patients had a Doppler ultrasound of the extremity in which their central venous catheter was placed at the end of induction or at presentation with symptoms suggesting thrombosis.
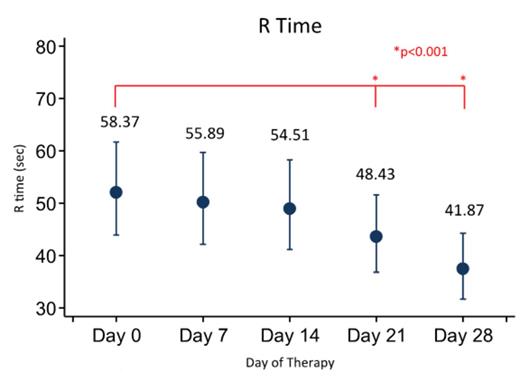
Results: Eighty patients have been enrolled (see Table 1 for demographics) with 72 being fully evaluable currently. Three (4.3%) patients developed symptomatic VTE and 7 (15%) patients had an asymptomatic VTE for an overall incidence of VTE of 21.7%. We demonstrate that R time gradually increases over time from baseline during induction therapy. Additionally, maximum amplitude (MA) is initially diminished, but then increases with time. There were no statistically significant differences in VTE incidence between males and females, different age groups (though there was a trend towards increased incidence for older children), or CVC type (none of the patients with an implanted port had a VTE). Interestingly, MA did not seem to be associated with platelet count.
Conclusion: Patients in this prospective study of patients with newly diagnosed ALL during induction at a single institution show rates of thrombosis in patients with ALL similar to that in the literature. Furthermore, TEG results demonstrated a shortening of the clotting time (R) and an increase in the clot rigidity (MA) during induction. Though not statistically significant, older children and patients with HR-ALL were more likely to have a VTE. Additional analyses will be performed to investigate the ability of the TEG, as well as the CBC and markers of thrombin generation, in predicting the development of VTE in these patients.
Patient Demographics
| Patient Demographics . | . | . | . | . | . |
|---|---|---|---|---|---|
| Gender | Female | 33 (46%) | |||
| Male | 38 (54%) | ||||
| Age | < 5 years | 28 (39%) | |||
| 9-12 years | 26 (37%) | ||||
| 10+ years | 17 (24%) | ||||
| Patient VTE classification | Symptomatic VTE | 3 (4%) | |||
| Asymptomatic VTE | 12 (17%) | ||||
| No VTE | 56 (79%) | ||||
| Demographics by CVC Type | Type of CVC | ||||
| PICC | Port-a-Cath | Total | |||
| Gender | Female | 35 (45%) | 3 (100%) | 38 (48%) | |
| Male | 42 (55%) | 0 (0%) | 42 (53%) | ||
| Age | < 5 years | 29 (38%) | 2 (67%) | 31 (39%) | |
| 9-12 years | 30 (39%) | 0 (0%) | 30 (38%) | ||
| 10+ years | 18 (23%) | 1 (33%) | 19 (24%) | ||
| Type of ALL | SR-ALL | 54 (70%) | 0 (0%) | 54 (68%) | |
| HR-ALL | 23 (30%) | 2 (67%) | 25 (31%) | ||
| T-ALL | 0 (0%) | 1 (33%) | 1 (1%) | ||
| Patient VTE classification | Symptomatic VTE | 3 (4%) | 0 (0%) | 3 (4%) | |
| Asymptomatic VTE | 12 (17%) | 0 (0%) | 12 (17%) | ||
| No VTE | 54 (78%) | 2 (100%) | 56 (79%) | ||
| Patient VTE classification | |||||
| Demographics by VTE | Symptomatic VTE | Asymptomatic VTE | No VTE | Total | |
| Gender | Female | 1 (33%) | 7 (58%) | 25 (45%) | 33 (46%) |
| Male | 2 (67%) | 5 (42%) | 31 (55%) | 38 (54%) | |
| Age categories | < 5 years | 1 (33%) | 4 (33%) | 23 (41%) | 28 (39%) |
| 9-12 years | 0 (0%) | 3 (25%) | 23 (41%) | 26 (37%) | |
| 10+ years | 2 (67%) | 5 (42%) | 10 (18%) | 17 (24%) | |
| Type of ALL | SR-ALL | 1 (33%) | 7 (58%) | 39 (70%) | 47 (66%) |
| HR-ALL | 2 (67%) | 5 (42%) | 16 (29%) | 23 (32%) | |
| T-ALL | 0 (0%) | 0 (0%) | 1 (2%) | 1 (1%) | |
| Patient Demographics . | . | . | . | . | . |
|---|---|---|---|---|---|
| Gender | Female | 33 (46%) | |||
| Male | 38 (54%) | ||||
| Age | < 5 years | 28 (39%) | |||
| 9-12 years | 26 (37%) | ||||
| 10+ years | 17 (24%) | ||||
| Patient VTE classification | Symptomatic VTE | 3 (4%) | |||
| Asymptomatic VTE | 12 (17%) | ||||
| No VTE | 56 (79%) | ||||
| Demographics by CVC Type | Type of CVC | ||||
| PICC | Port-a-Cath | Total | |||
| Gender | Female | 35 (45%) | 3 (100%) | 38 (48%) | |
| Male | 42 (55%) | 0 (0%) | 42 (53%) | ||
| Age | < 5 years | 29 (38%) | 2 (67%) | 31 (39%) | |
| 9-12 years | 30 (39%) | 0 (0%) | 30 (38%) | ||
| 10+ years | 18 (23%) | 1 (33%) | 19 (24%) | ||
| Type of ALL | SR-ALL | 54 (70%) | 0 (0%) | 54 (68%) | |
| HR-ALL | 23 (30%) | 2 (67%) | 25 (31%) | ||
| T-ALL | 0 (0%) | 1 (33%) | 1 (1%) | ||
| Patient VTE classification | Symptomatic VTE | 3 (4%) | 0 (0%) | 3 (4%) | |
| Asymptomatic VTE | 12 (17%) | 0 (0%) | 12 (17%) | ||
| No VTE | 54 (78%) | 2 (100%) | 56 (79%) | ||
| Patient VTE classification | |||||
| Demographics by VTE | Symptomatic VTE | Asymptomatic VTE | No VTE | Total | |
| Gender | Female | 1 (33%) | 7 (58%) | 25 (45%) | 33 (46%) |
| Male | 2 (67%) | 5 (42%) | 31 (55%) | 38 (54%) | |
| Age categories | < 5 years | 1 (33%) | 4 (33%) | 23 (41%) | 28 (39%) |
| 9-12 years | 0 (0%) | 3 (25%) | 23 (41%) | 26 (37%) | |
| 10+ years | 2 (67%) | 5 (42%) | 10 (18%) | 17 (24%) | |
| Type of ALL | SR-ALL | 1 (33%) | 7 (58%) | 39 (70%) | 47 (66%) |
| HR-ALL | 2 (67%) | 5 (42%) | 16 (29%) | 23 (32%) | |
| T-ALL | 0 (0%) | 0 (0%) | 1 (2%) | 1 (1%) | |
Ko:Alexion: Honoraria; NovoNordisk: Consultancy. Young:Bayer: Consultancy; Kedrion: Consultancy; Novo Nordisk: Consultancy, Honoraria; Baxter: Consultancy; Biogen Idec: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal