Abstract
Background:
Venous thromboembolism (VTE) is a common complication in cancer patients. Anticoagulation (AC) remains the standard of care for treatment of cancer-associated VTE, but inferior vena cava filters (IVCF) are often used in place of or in addition to AC for various indications. Data are limited addressing the role for IVCF in cancer patients. We examine the efficacy of IVCF in a large cohort of cancer patients with prior pulmonary embolism (PE), to assess for rates recurrent VTE within 1-yr of initial VTE and overall survival (OS).
Methods:
This is a retrospective, single institution study. The study population comprised of all patients diagnosed with a PE at Memorial Sloan Kettering Cancer Center (MSKCC) from 2008-2009 (N= 1272). Patients had diverse primary tumors including lung (N= 246), colorectal (N= 139), gynecologic (N= 130), and breast (N= 103). The majority of patients (96%) were placed on therapeutic AC at time of diagnosis of PE (within 7 days of PE diagnosis). Subsequent interruptions in AC could not be captured.
The cumulative incidence for recurrent DVT/PE was calculated from date of initial PE diagnosis to date of recurrent DVT/PE, death, or last followup. Deaths without recurrent DVT/PE were considered a competing event. Patients alive without event are censored at 12 months. Gray's k-test and Fine & Gray regression were used in analyzing the relationship between recurrent DVT/PE and IVCF placement. OS was calculated from date of initial PE diagnosis to date of death or last follow up. Log-rank test was used to compare OS by IVCF placement.
Results:
25% (N=317) of the 1272 cancer-associated PE cohort had IVCF placed, either within 30 days following the initial PE (N=274), or prior to the initial PE (N=43). These 317 patients are compared to the 955 patients without IVCF. The indications for IVCF placement included AC contraindicated (39%), pre-operative (16%), AC failure (16%), poor cardiopulmonary reserve (6%) or indication unclear (23%).
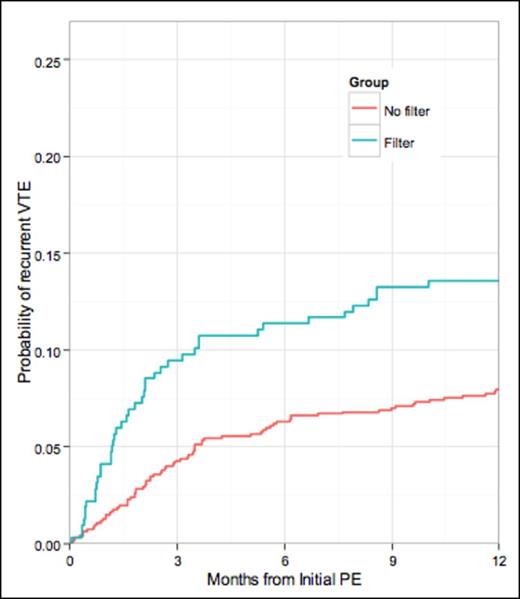
Composite 12-month rate of all recurrent VTE rate was higher with IVCF (14%) than non-IVCF (8%), (p = 0.016). Adjusting for whether patients were placed on AC at time of initial PE, this difference remained statistically significant with p = 0.014. After adjusting for AC, the risk of recurrent PE was similar between the IVCF cohort (5%) and non-IVCF (4%), (p = 0.43), but the risk of DVT was significantly higher in the IVCF group, 9% versus 5% (p = 0.014).
Median OS for IVCF patients was 7.4 months, versus 13.5 months in non-IVCF patients, (p = <0.001 by log-rank testing). Median time from IVCF placement to death was 3.6 months (range 0.07-88.4 months) with 62 IVC filters placed within 1 month before death (14%).
Radiographic evidence IVCF thrombosis was observed in 13% (N= 42). Interestingly, 55% (N=23) of these patients were on AC at time of IVCF thrombosis, suggesting that AC is not fully protective against this complication. Other complications include filter projection to outside IVC walls (N=6), which in 1 case was associated with erosive changes to L3 vertebral body from the IVCF strut, and filter migration to right renal vein (N=1).
Conclusions:
In this population of cancer patients with newly diagnosed PE, IVCF use was common and was associated with poorer OS, higher rates of recurrent DVT and similar rates of recurrent PE, even when adjusting for AC at initial PE diagnosis. Limitations include the retrospective, non-randomized nature of this study. Further, we were unable to account for AC interruptions following initial PE diagnosis, which likely influenced the recurrent VTE rates. The poorer survival with IVCF may reflect poorer performance status and generally sicker nature associated with patients undergoing IVCF placement. Still, the absence of significant reduction in PE rates with the IVCF is noteworthy. A putative partial protective effect of IVCF on PE rates may have been counterbalanced by a higher VTE risk associated with poorer performance status in those receiving the IVCF. However, this large cohort study should give pause before placement of IVCF in many cancer patients, especially with advanced stage disease.
Gray's k-test comparing the incidence curves by filter group is 0.0162. The p-value for filter (adjusting for AC at initial PE) is 0.014.
Gray's k-test comparing the incidence curves by filter group is 0.0162. The p-value for filter (adjusting for AC at initial PE) is 0.014.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal