In this issue of Blood, Liang et al identify a critical role of coagulation factor V in host inflammation response to infections that could guide our exploration for better therapy for sepsis.1
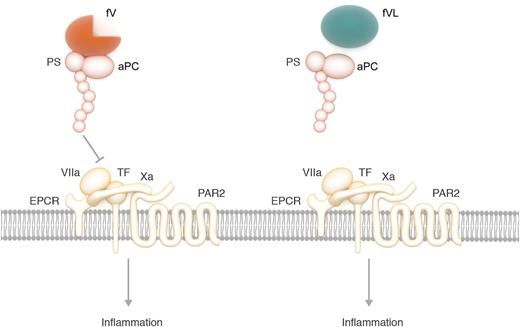
aPC with cofactor protein S (PS) and fV inhibits the EPCR-dependent activation of the inflammatory PAR2 signaling by destabilizing the ternary TF-VIIa-Xa complex. Without the R506 cleavage site, fV Leiden (FVL) loses its cofactor activity to facilitate aPC inhibition of the inflammatory signaling. Professional illustration by Somersault18:24.
aPC with cofactor protein S (PS) and fV inhibits the EPCR-dependent activation of the inflammatory PAR2 signaling by destabilizing the ternary TF-VIIa-Xa complex. Without the R506 cleavage site, fV Leiden (FVL) loses its cofactor activity to facilitate aPC inhibition of the inflammatory signaling. Professional illustration by Somersault18:24.
Severe sepsis is a common, life-threatening infection resulting from a number of pathogens. Understanding the complex host inflammation and coagulation response during sepsis is key to finding a solution to this severe disease. The uncontrolled inflammatory response by the host to the infection results in multiorgan failure and death in sepsis. Numerous studies have been performed to study the effects of anti-inflammatory and antithrombotic agents on sepsis outcomes. Only human recombinant activated protein C (aPC) demonstrated efficacy in clinical trials for improving the 28-day mortality rate of septic shock patients in 2001.2 However, drotrecogin alfa (activated; recombinant human aPC) was withdrawn from the market in October 2011.3 A multicenter investigator-led trial found no evidence for benefit of recombinant human aPC in adults with septic shock.4 aPC cleaves activated factor Va and factor VIIIa and also demonstrates anti-inflammatory and cytoprotective effects.5 Patients with severe sepsis who were heterozygote carriers of the factor V (fV) Leiden mutation, a prothrombotic mutation of fV resistant to aPC cleavage, demonstrated an improved survival rate.6 These findings suggest that the roles of fV in aPC function are complicated and merit further exploration.
Liang et al provide evidence that fV is a cofactor of the aPC anti-inflammatory function. They demonstrate that the anticoagulant activity of aPC toward fVa could even have an adverse effect on sepsis outcome. Furthermore, aPC had no efficacy in sepsis in homozygote fV Leiden mice. Lack of therapeutic benefit in fV Leiden homozygotes was due not to increased thrombin generation, but to lack of wild-type fV to facilitate the anti-inflammatory function of aPC. In the presence of wild-type fV, aPC can suppress the gene expression profile change induced by lipopolysaccharide (LPS), whereas it fails to do so in the presence of fV Leiden. Analysis of the gene expression signature suggested that aPC and fV mediated this LPS-induced transcription program through the endothelial protein C receptor (EPCR)-dependent activation of protease-activated receptor 2 (PAR2) by the ternary tissue factor (TF)-VIIa-Xa complex.7 Stabilizing the TF-VIIa-Xa complex with nematode anticoagulant protein C2 (NapC2) abolished aPC’s inhibitory activity against gene expression, suggesting that aPC inhibits TF-EPCR-PAR2 by destabilizing the TF-VIIa-Xa complex. By testing several function-selective aPC variants and recombinant fV mutants, Liang et al found that inhibition of TF-EPCR-PAR2 signaling by aPC required protein S, the fV B domain, and the intact R506 cleavage site.
In summary, the results of Liang et al suggest that fV served as a critical cofactor with protein S for aPC to destabilize the ternary TF-VIIa-Xa complex, leading to inhibition of the EPCR-dependent activation of inflammatory PAR2 signaling (see figure). This anti-inflammatory cofactor activity of fV required the B domain and the intact R506 cleavage site. In the presence of fV Leiden and lack of wild-type fV, aPC lost anti-inflammatory activity (see figure). As a result, aPC lost its therapeutic benefit in fV Leiden homozygotes.
This study provides novel insights into the complex interplay of inflammation and coagulation in sepsis. Inflammatory and thrombotic complications are interlinked during infections.8 The authors point out the importance of balancing the anticoagulant and anti-inflammatory activity of aPC in designing sepsis treatment to maximize the benefit for the patients. The unique dual anticoagulant and anti-inflammatory functions of aPC have been much of the focus of studies in our effort to find therapies for sepsis. The current study demonstrates that fV is not a passive player in aPC’s anti-inflammatory function. Not only is fV a substrate and cofactor of aPC anticoagulant function, it is also an active cofactor of its anti-inflammatory function.
This study thus provides some intriguing clues to understand the survival advantage of fV Leiden heterozygotes among sepsis patients. Could it be due to the combination of partially suppressing aPC’s anticoagulant activity while still maintaining fV’s cofactor activity for aPC’s anti-inflammatory activity? Conversely, the authors carefully point out that fV Leiden carriership had no effect on several infectious diseases.9,10 It remains to be investigated whether this fV-dependent aPC anti-inflammatory activity is disease context and pathogen specific. They also suggest that the fV-dependent inhibition of TF-EPCR-PAR2 signaling was not the only factor contributing to sepsis mortality reduction by aPC. It should be noted that the current study was performed using therapeutically administrated aPC, which may use a different mechanism of action than endogenous aPC.
More studies are needed to identify additional players in host inflammation and coagulation response during sepsis. Nevertheless, this progress in our understanding of the molecular mechanism of host inflammation and coagulation response to infections offers great encouragement in our search for sepsis therapy.
Conflict-of-interest disclosure: H.S. owns stock in Nanova, Inc.