In this issue of Blood, Zhu et al have established, in human blood, that factor XIa and polyphosphate make significant contributions to thrombus formation.1 This makes these molecules good targets for therapeutic intervention.
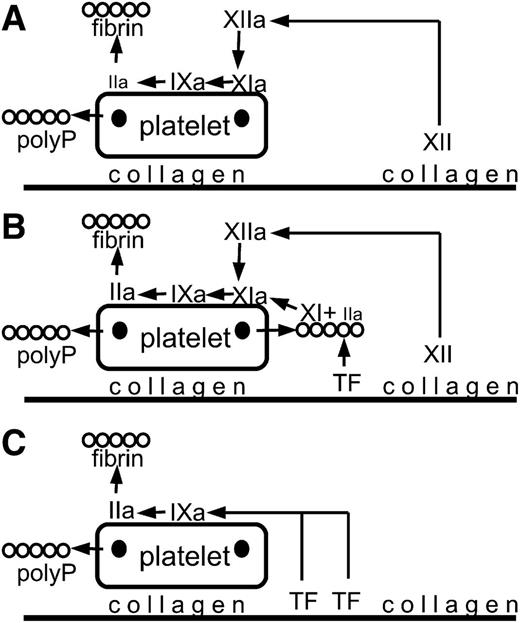
A broad overview of the components that are subject to inhibition under 3 sets of conditions (many of the coagulation steps are omitted). For an accurate analysis, see supplemental Figure 6 in the article by Zhu et al that begins on page 1494. Platelets adhere to collagen and are partially activated releasing polyphosphate from dense granules (dark spots on the platelets). During fibrin formation, fibrin structure is altered by association with polyphosphate, leading to a structure that is more resistant to lysis. (A) Factor XII is activated to factor XIIa. Factor XIIa activates factor XI to factor XIa, which activates factor IX to factor IXa, leading to thrombin (IIa) generation and fibrin formation. (B) Tissue factor initiates the generation of a small amount of thrombin (IIa) that binds to polyphosphate and activates factor XI to factor XIa. Additional factor XIa is generated from factor XIIa formed by contact activation. Factor XIa activates factor IX to factor IXa, leading to thrombin (IIa) generation and fibrin formation. (C) At high tissue factor, sufficient factor IX is activated to factor IXa to drive thrombin (IIa) generation and fibrin formation. polyP, polyphosphate; TF, tissue factor.
A broad overview of the components that are subject to inhibition under 3 sets of conditions (many of the coagulation steps are omitted). For an accurate analysis, see supplemental Figure 6 in the article by Zhu et al that begins on page 1494. Platelets adhere to collagen and are partially activated releasing polyphosphate from dense granules (dark spots on the platelets). During fibrin formation, fibrin structure is altered by association with polyphosphate, leading to a structure that is more resistant to lysis. (A) Factor XII is activated to factor XIIa. Factor XIIa activates factor XI to factor XIa, which activates factor IX to factor IXa, leading to thrombin (IIa) generation and fibrin formation. (B) Tissue factor initiates the generation of a small amount of thrombin (IIa) that binds to polyphosphate and activates factor XI to factor XIa. Additional factor XIa is generated from factor XIIa formed by contact activation. Factor XIa activates factor IX to factor IXa, leading to thrombin (IIa) generation and fibrin formation. (C) At high tissue factor, sufficient factor IX is activated to factor IXa to drive thrombin (IIa) generation and fibrin formation. polyP, polyphosphate; TF, tissue factor.
The emphasis on human blood is important, as many thrombosis studies have been conducted in mice. Mouse studies take advantage of 2 things: (1) robust imaging tools that allow in vivo thrombus formation to be monitored and quantified, and (2) genetic manipulation of the mice to generate mechanistic information. However, there are data to suggest that mice may have significant differences in thrombus formation relative to primates and humans.2 Thus, it is critical to test potential antithrombotic mechanisms in human blood.
Directed antithrombotics in current use are targeted inhibitors of thrombin and factor Xa. However, Zhu et al went in a different direction and studied the role of factor XI. Factor XI is an especially interesting molecule, because it lies at the critical junction of the classical contact pathway (factor XIIa and high-molecular-weight kininogen activation of factor XI) and the platelet-driven thrombin feedback amplification loop. This feedback was suggested by studies showing that sulfated glycans could promote thrombin activation of factor XI.3,4 Subsequent work provided a physiological basis by showing that activated platelets could sustain this reaction.5,6 The laboratory of Dr Morrissey established the mechanism of this activation by showing that polyphosphate released from platelet-dense granules was the agent that promoted thrombin activation of factor XI.7
A logical extension of those studies on the biochemistry of polyphosphate is to examine the antithrombotic properties of agents that target factor XI and polyphosphate. In this study, Zhu et al used sophisticated flow models of thrombosis to generate nuanced results.1 They used whole blood to incorporate the critical contribution of platelets. They included the role of flow by passing the blood over collagen to give platelet adherence and contact activation. They studied the results of 3 inhibitors: (1) an antibody that selectively blocks factor XIIa activation of factor XI (without interfering with thrombin activation of factor XI), (2) an antibody that blocks factor XIa activation of factor IX, and (3) a compound that binds and neutralizes polyphosphate (see figure). With these 3 inhibitors, they used low, medium, and high tissue factor to modulate the procoagulant signal. The study is complicated by the fact that merely drawing blood can result in nonphysiological contact activation. Zhu et al get around this by using a small amount of an inhibitor of factor XIIa. This allows them to suppress background effects while continuing to measure the contribution of factor XII and the contact pathway.
Unsurprisingly, in the absence of tissue factor, platelet surface factor IX activation was purely driven by contact factors. Similarly, at high tissue factor, sufficient factor IXa was generated by a tissue factor reaction in which the contribution of contact factor was less significant. At intermediate TF levels, the inhibitors of factor XI activation and activity slowed thrombus formation and reduced thrombus size. This effect was not related to platelet accumulation into the thrombus but was specific for thrombin generation and fibrin formation.
Perhaps more interesting, Zhu et al showed that polyphosphate played a role in more than just thrombin generation through feedback activation of factor XI. Polyphosphate directly interacted with fibrin in a way that made the thrombus less susceptible to lysis by fibrinolytic agents. Blocking polyphosphate reduced thrombus stability and increased lysis of the fibrin clot. All of this suggests that polyphosphate is an intriguing target for antithrombotic agents. Such an antithrombotic would not only reduce thrombin generation but also alter fibrin structure to promote lysis of any thrombus that does form.
One of the holy grails of antithrombotic therapy is to have agents that are effective without increasing the risk of bleeding. Zhu et al discuss the data suggesting that the contribution of the contact pathway to hemostasis is to augment the existing platelet-driven thrombin generation. In this study, Zhu et al significantly advance our understanding of possible contributions of the contact pathway to thrombus formation. If, as this study suggests, factor XI and polyphosphate have a greater contribution to thrombus formation than hemostasis in human blood, then those molecules make appealing targets for novel antithrombotic agents.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal