Key Points
IRF8 does not instruct monocytic lineage specification in oligopotent granulocyte-monocyte progenitors.
IRF8 regulates the survival and differentiation of lineage-committed progenitors to promote monocyte and suppress neutrophil production.
Abstract
Interferon regulatory factor 8 (IRF8) is a key regulator of myelopoiesis in mice and humans. IRF8-deficient mice exhibit increased neutrophil numbers but defective monocyte and dendritic cell (DC) production. It has therefore been hypothesized that IRF8 regulates granulocyte vs monocyte/DC lineage commitment by oligopotent progenitors. Alternatively, IRF8 could control the differentiation of lineage-committed progenitors. In this study, we defined the role of IRF8 in lineage commitment and neutrophil vs monocyte differentiation using a novel sorting strategy that for the first time allows us to separate oligopotent granulocyte-monocyte progenitors (GMPs) and their lineage-committed progeny: granulocyte progenitors (GPs) and monocyte progenitors (MPs). We show that IRF8 is highly expressed by both GPs and MPs, but not GMPs, and is not required for GP or MP production by GMPs. In fact, IRF8-deficient mice have more GPs and MPs. This is not due to IRF8-mediated suppression of GP and MP production by GMPs, but rather to selective effects in GPs and MPs. We identify roles for IRF8 in regulating progenitor survival and differentiation and preventing leukemic cell accumulation. Thus, IRF8 does not regulate granulocytic vs monocytic fate in GMPs, but instead acts downstream of lineage commitment to selectively control neutrophil and monocyte production.
Introduction
The transcription factor interferon regulatory factor 8 (IRF8, also known as ICSBP) is a key regulator of myeloid cell production and function. Mice lacking IRF8 have reduced numbers of monocytes and dendritic cells (DCs), have elevated neutrophil counts, exhibit defective antimicrobial immunity, and develop chronic myelogenous leukemia (CML)–like disease.1-9 In humans, IRF8 mutations are associated with immunodeficiency, and decreased IRF8 expression has been observed in both acute myelogenous leukemia (AML) and CML patients.10-12
Previous studies have shown that IRF8 promotes monocyte and DC production but limits neutrophil production.1,4,6-8,13-15 Thus, it is widely thought to act in oligopotent progenitors (common myeloid progenitors [CMPs], and/or granulocyte-monocyte progenitors [GMPs]) to instruct the adoption of monocyte/DC fate and suppress granulocyte fate. Alternatively, IRF8 could act in lineage-committed progenitors (granulocyte progenitors [GPs], monocyte progenitors [MPs], and DC progenitors) to selectively promote or limit their survival, proliferation, and/or differentiation. It has not previously been possible to discriminate between these “instruction” and “selection” mechanisms, in large part because markers have not been defined to separate oligopotent GMPs from their lineage-committed progeny: GPs and MPs.
Hematopoietic progenitors are defined by their potential to produce cells of specific hematopoietic lineages. Oligopotent GMPs capable of producing granulocytes (including neutrophils) and monocytes, but not lymphocytes, erythrocytes, and megakaryocytes, form colonies comprising neutrophils and macrophages (but not erythrocytes) in methylcellulose cultures supplemented with hematopoietic cytokines. These granulocyte-monocyte colony-forming units (GM-CFUs) are found in the Lin−c-Kit+Sca-1− (LKS−) CD34+FcγRhi fraction of mouse bone marrow and the Lin−IL-3RaloCD45RA+ fraction of adult human bone marrow.16,17
However, the LKS−CD34+FcγRhi and Lin−IL-3RaloCD45RA+ fractions are very heterogeneous. In addition to oligopotent GMPs, they also contain large proportions of progenitors that do not possess the ability to form both granulocytes and monocytes, but are instead committed to the production of one cell type or the other, that is, granulocyte-CFU (GPs) and monocyte-CFU (MPs).16,17 Thus, as noted, although IRF8 has been shown to be expressed by LKS−CD34+FcγRhi cells and to regulate neutrophil and monocyte production,6-8,18 it has not previously been possible to determine in which progenitors it acts and whether it uses “instruction” and/or “selection” mechanisms. It could favor monocyte production by instructing oligopotent GMPs to produce MPs, or perhaps selectively act in lineage-committed progenitors to promote MP differentiation and suppress GP differentiation, or it could act at both the oligopotent and lineage-committed progenitor stages.
In this study, we report the first strategy to separate oligopotent GMPs (Ly6C−) and lineage-committed GPs (Ly6C+CD115lo) and MPs (Ly6C+CD115hi) from the LKS−CD34+FcγRhi fraction of mouse bone marrow. This has now enabled us to demonstrate that IRF8 is highly expressed by GPs and MPs but not oligopotent GMPs, and is not required for GP or MP production by GMPs. Instead, we show that it acts in the lineage-committed progenitors to selectively limit neutrophil production by GPs and promote monocyte production by MPs.
Materials and methods
Mice
Wild-type C57BL/6 mice (CD45.2), congenic wild-type CD45.1 mice (B6.SJL-PtprcaPepcb/BoyJ strain), and IRF8-deficient mice (all from The Jackson Laboratory) were maintained at Cedars-Sinai Medical Center; all procedures were performed with institutional animal care and use committee approval.
Cell staining for flow cytometry and FACS sorting
Antibodies used for flow cytometric analysis and fluorescence-activated cell sorting (FACS) were: CD34 (RAM34) and FcγR (93) from eBioscience; c-Kit (2B8), Ly6C (HK1.4), Ly6G (1A8), Gr-1 (RB6-8C5), CD11b (M1/70), F4/80 (BM8), CD115 (AFS98), CD45.1 (A20), and CD45.2 (104) from BioLegend; c-Kit (3C1) and Sca-1 (D7) from Miltenyi Biotec; IRF8 (C-19) and c/EBPα (14AA) from Santa Cruz Biotechnology; and PU.1 (9G7) from Cell Signaling Technology. Where possible, nonspecific antibody binding was prevented by prior incubation with Fc block (anti-CD16/anti-CD32). For identification of progenitors by FcγR expression, cells were stained for FcγR prior to staining with other antibodies. A cell fixation and permeabilization kit (Invitrogen) was used for intracellular staining. An LSRFortessa (BD Biosciences) was used for flow cytometry and data were analyzed with FlowJo.
Isolation of mouse bone marrow progenitors, neutrophils, and monocytes
Mouse progenitors were isolated from bone marrow (femurs and tibias) by a combination of magnetic and fluorescence sorting. Lineage marker negative cells (Lin−) were first separated using a magnetic-activated cell sorting (MACS) lineage cell depletion kit (containing antibodies against CD5, CD11b, Gr-1, 7-4, and Ter-119) and an autoMACS Separator (both from Miltenyi Biotec). Lin− cells were further fractionated using a FACSAria III cell sorter (BD Biosciences) to isolate stem and progenitor cell subpopulations as outlined in the text and figure legends. Neutrophils (CD11b+Ly6GhiLy6Cint) and monocytes (CD11b+Ly6G−Ly6Chi) were also purified from mouse bone marrow by FACS sorting.
In vitro progenitor differentiation
Liquid cultures
Isolated progenitors were plated in media supplemented with granulocyte–colony-stimulating factor (G-CSF) (50 ng/mL), interleukin-3 (IL-3) (10 ng/mL) + IL-6 (20 ng/mL), macrophage-CSF (M-CSF) (50 ng/mL), or granulocyte macrophage–CSF (GM-CSF) (20 ng/mL) (PeproTech). At the indicated time points, cells were analyzed by flow cytometry to identify progenitors, neutrophils, monocytes, and macrophages.
Methylcellulose cultures
To evaluate progenitor hematopoietic potential, 1 × 103 cells were plated in triplicate in MethoCult GFM3434 (components include insulin, transferrin, stem cell factor, IL-3, IL-6, erythropoietin; StemCell Technologies). Colonies were counted and identified 7 days later. For some assays, total cells harvested from 1° cultures were counted and plated at equal numbers in fresh methylcellulose (2° culture); colonies were then counted after a further 7-day incubation.
In vivo progenitor differentiation
Wild-type CD45.2 progenitors were IV injected (1-2 × 105 cells per mouse in 100 μL of phosphate-buffered saline) into nonirradiated CD45.1 recipient mice on day 0. Mice were sacrificed 2 to 7 days later, and spleens were harvested for flow cytometry following erythrocyte lysis with ammonium chloride. Cells were stained for CD45.1, CD45.2, CD11b, Ly6C, Ly6G, and F4/80 for neutrophil, monocyte, and macrophage identification, and CD45.1, CD45.2, c-Kit, FcγR, and Ly6C for progenitor identification. For GMP-Ly6C+CD115lo/hi experiments, splenic cells were MACS sorted (CD45.1 depletion) prior to flow cytometric analysis to enrich for CD45.2+ donor-derived cells.
Western blotting
Whole-cell lysates were separated using the Novex NuPAGE gel electrophoresis system (Invitrogen) and proteins were transferred on to Immobilon-FL polyvinylidene difluoride (PVDF) membranes (Millipore). Blots were probed with antibodies against IRF8 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology) followed by infrared dye-conjugated secondary antibodies (LI-COR), and bands were visualized on an Odyssey imaging system (LI-COR).
Apoptosis and proliferation assays
Apoptotic cells were detected by flow cytometry following staining with fluorescein isothiocyanate–Annexin V (BioLegend) and the appropriate antibodies for cell identification. To assess proliferation, sorted cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes) according to the manufacturer’s instructions, and washed thoroughly prior to culture. Cells were then identified by staining with fluorescent antibodies; CFSE dilution was analyzed by flow cytometry.
May-Grünwald Giemsa staining
Cytospin preparations of sorted cells were stained with May-Grünwald Giemsa stain (American MasterTech) according to the manufacturer’s instructions, mounted in DPX mountant for histology (Sigma-Aldrich), and imaged on a Leitz Laborlux 12 microscope (Zeiss) using a 16× objective. Bright-field micrographs of the cells were acquired with a ProgRes 10plus camera using ProgRes CapturePro 2.7 acquisition software (Jenoptik).
Statistics
Statistical analysis was performed using Student t tests.
Results
Separate isolation of oligopotent GMPs and lineage-committed GPs and MPs
While studying macrophage differentiation from mouse bone marrow progenitors (Lin− cells), we noticed that Ly6C, which is widely considered to be a marker of inflammatory monocytes, is also expressed by a subpopulation of progenitor cells (supplemental Figure 1A, available on the Blood Web site). This is perhaps surprising, given that the antibody cocktail used to isolate the Lin− progenitors by depleting differentiated cells contains the Gr-1 antibody, which detects Ly6G and to a lesser extent Ly6C. However, staining of total bone marrow cells revealed that this antibody fails to detect a population of Ly6C-expressing bone marrow cells, which are retained and enriched following depletion with the lineage marker antibody cocktail (supplemental Figure 1B).
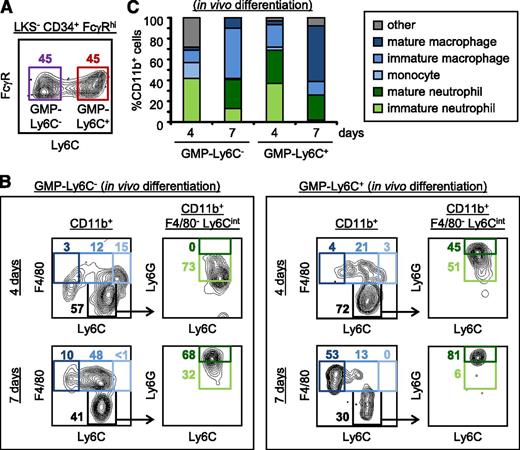
Further investigation revealed that Ly6C+ cells are found in the LKS−CD34+FcγRhi subset (Figure 1A; supplemental Figure 1C-D). LKS−CD34+FcγRhi cells, which yield granulocytes and/or monocytes, are known as GMPs,16 so we subsequently refer to the Ly6C− and Ly6C+ subpopulations as GMP-Ly6C− and GMP-Ly6C+ cells, respectively. We verified that both the GMP-Ly6C− and GMP-Ly6C+ subsets were c-Kithi (although the level of c-Kit expression by GMP-Ly6C+ cells was slightly lower than GMP-Ly6C− cells) and that neither population expressed the myeloid cell marker CD11b, the neutrophil marker Ly6G, or the Gr-1 epitope (supplemental Figure 1E). We therefore FACS sorted the GMP-Ly6C− and GMP-Ly6C+ subsets (Figure 1A) to determine their lineage potential.
Ly6C expression marks a subset of mouse bone marrow GMPs. (A) Ly6C expression by LKS−CD34+FcγRhi cells (unsorted GMPs) was assessed by flow cytometry (see “Materials and methods” and supplemental Figure 1 for sorting and gating strategies). Data are representative of >3 independent experiments. (B-C) GMP-Ly6C− and GMP-Ly6C+ cells were FACS sorted from CD45.2 donor mouse Lin− cells and 200 000 cells were injected IV into congenic CD45.1 recipient mice (nonirradiated) on day 0. Spleens were harvested from recipient mice on days 4 and 7, and donor cell-derived (CD45.2+) neutrophils (green gates), monocytes, and macrophages (blue gates) were detected by flow cytometry (see supplemental Figure 4 for gating strategy). CD45.2+CD11b+ splenic cells are shown. Data presented are from 1 experiment that is representative of at least 3 independent experiments.
Ly6C expression marks a subset of mouse bone marrow GMPs. (A) Ly6C expression by LKS−CD34+FcγRhi cells (unsorted GMPs) was assessed by flow cytometry (see “Materials and methods” and supplemental Figure 1 for sorting and gating strategies). Data are representative of >3 independent experiments. (B-C) GMP-Ly6C− and GMP-Ly6C+ cells were FACS sorted from CD45.2 donor mouse Lin− cells and 200 000 cells were injected IV into congenic CD45.1 recipient mice (nonirradiated) on day 0. Spleens were harvested from recipient mice on days 4 and 7, and donor cell-derived (CD45.2+) neutrophils (green gates), monocytes, and macrophages (blue gates) were detected by flow cytometry (see supplemental Figure 4 for gating strategy). CD45.2+CD11b+ splenic cells are shown. Data presented are from 1 experiment that is representative of at least 3 independent experiments.
We first assessed the ability of the GMP-Ly6C− and GMP-Ly6C+ fractions to produce neutrophils and monocytes/macrophages. Both populations yielded large numbers of neutrophils in in vitro liquid cultures supplemented with G-CSF or IL-3 + IL-6, and macrophages in M-CSF cultures (supplemental Figures 2-3). To assess in vivo differentiation, we injected progenitors from donor mice (CD45.2) into nonirradiated congenic (CD45.1) recipient mice and monitored the appearance of neutrophils and macrophages in the spleen. Both the Ly6C− and the Ly6C+ donor cells yielded neutrophils and macrophages in vivo (Figure 1B-C; supplemental Figure 4).
Notably, in both the in vitro cultures and the in vivo experiments, GMP-Ly6C+ cells yielded neutrophils and macrophages at earlier time points than GMP-Ly6C− cells (Figure 1B-C; supplemental Figure 3). These data suggested that the GMP-Ly6C+ cells could represent a later progenitor stage than the GMP-Ly6C− cells. We therefore examined whether GMP-Ly6C− cells produce GMP-Ly6C+ cells. In liquid cultures with G-CSF, IL-3 + IL-6, or M-CSF, the GMP-Ly6C+ cells rapidly lost the progenitor marker c-Kit and gained CD11b as they differentiated toward neutrophils and macrophages; in contrast, GMP-Ly6C− cells first yielded GMP-Ly6C+ cells prior to the appearance of CD11b+ cells (Figure 2A; supplemental Figures 5-6). Similarly, GMP-Ly6C+ donor cells rapidly lost c-Kit and then gained CD11b in vivo, whereas GMP-Ly6C− donor cells yielded GMP-Ly6C+ cells prior to losing c-Kit and gaining CD11b (supplemental Figure 7).
GMP-Ly6C+ cells are the lineage-committed progeny (MPs and GPs) of oligopotent GMP-Ly6C− cells (GMPs). (A-B) Ten thousand GMP-Ly6C− or GMP-Ly6C+ cells per well (A) or 10 000 LKS−CD34+FcγRlo CMPs per well (B) were cultured in liquid media containing 10 ng/mL IL-3 and 20 ng/mL IL-6, and cultures were analyzed by flow cytometry at the indicated time points to monitor for the presence of CMPs, GMP-Ly6C−, GMP-Ly6C+, and CD11b+ cells (see supplemental Figure 5 for gating strategy). Data presented are from 1 experiment that is representative of at least 3 independent experiments. (C-G) Methylcellulose cultures were performed using MethoCult GFM3434 (StemCell Technologies). For primary cultures (C-F), unsorted GMPs (LKS−CD34+FcγRhi cells), GMP-Ly6C−, and GMP-Ly6C+ cells were isolated from mouse bone marrow and 1000 cells per well were plated in methylcellulose media. (C-D) Colonies were counted and identified 7 days later: GM, mixed granulocyte/monocyte; G, pure granulocyte; M, pure monocyte colonies. Data are presented as mean plus standard deviation of triplicate culture and are representative of 3 independent experiments. (E-F) All cells were harvested from the 7-day primary cultures, colonies were dissociated, and cells were counted to permit assessment of average colony size (total harvested cells per number of colonies) (E); c-Kit+ cells among the progeny were identified by flow cytometry (F). Data are presented as mean plus standard deviation of 3 independent experiments. (G) The progeny of 7-day primary cultures were plated in fresh methylcellulose (50 000 cells per well) for secondary culture, and colonies were counted after a further 7 days of culture. Data are presented as mean plus standard deviation of 3 independent experiments. Statistical significance was assessed by the Student t test (*P < .05, **P < .01). n.s., not significant.
GMP-Ly6C+ cells are the lineage-committed progeny (MPs and GPs) of oligopotent GMP-Ly6C− cells (GMPs). (A-B) Ten thousand GMP-Ly6C− or GMP-Ly6C+ cells per well (A) or 10 000 LKS−CD34+FcγRlo CMPs per well (B) were cultured in liquid media containing 10 ng/mL IL-3 and 20 ng/mL IL-6, and cultures were analyzed by flow cytometry at the indicated time points to monitor for the presence of CMPs, GMP-Ly6C−, GMP-Ly6C+, and CD11b+ cells (see supplemental Figure 5 for gating strategy). Data presented are from 1 experiment that is representative of at least 3 independent experiments. (C-G) Methylcellulose cultures were performed using MethoCult GFM3434 (StemCell Technologies). For primary cultures (C-F), unsorted GMPs (LKS−CD34+FcγRhi cells), GMP-Ly6C−, and GMP-Ly6C+ cells were isolated from mouse bone marrow and 1000 cells per well were plated in methylcellulose media. (C-D) Colonies were counted and identified 7 days later: GM, mixed granulocyte/monocyte; G, pure granulocyte; M, pure monocyte colonies. Data are presented as mean plus standard deviation of triplicate culture and are representative of 3 independent experiments. (E-F) All cells were harvested from the 7-day primary cultures, colonies were dissociated, and cells were counted to permit assessment of average colony size (total harvested cells per number of colonies) (E); c-Kit+ cells among the progeny were identified by flow cytometry (F). Data are presented as mean plus standard deviation of 3 independent experiments. (G) The progeny of 7-day primary cultures were plated in fresh methylcellulose (50 000 cells per well) for secondary culture, and colonies were counted after a further 7 days of culture. Data are presented as mean plus standard deviation of 3 independent experiments. Statistical significance was assessed by the Student t test (*P < .05, **P < .01). n.s., not significant.
We also examined the production of GMP-Ly6C− and GMP-Ly6C+ cells by CMPs. Consistent with the sequential production of GMP-Ly6C− cells and then GMP-Ly6C+ cells, CMPs yielded GMP-Ly6C− cells at early time points, prior to the accumulation of GMP-Ly6C+ cells, followed by the appearance of c-Kit−CD11b+ myeloid cells (Figure 2B; supplemental Figure 6B).
To determine whether the Ly6C− and Ly6C+ subsets represent oligopotent (GMP) or lineage-committed progenitor (GP and/or MP) populations, we assessed their colony-forming capacity in methylcellulose. Consistent with previous reports that the LKS−CD34+FcγRhi fraction comprises oligopotent GMPs (CFU-GM, which form colonies containing both granulocytes and monocytes/macrophages in methylcellulose cultures) as well as lineage-committed GPs (CFU-G, which produce only granulocytes) and MPs (CFU-M, which produce only monocytes/macrophages),16 we observed <40% mixed GM colonies upon culture of unfractionated LKS−CD34+FcγRhi cells (Figure 2C). GMP-Ly6C− cells on the other hand predominantly yielded granulocyte-macrophage colonies (Figure 2C), which enables us to identify them as oligopotent GMPs. In contrast, the GMP-Ly6C+ cells produced pure granulocyte colonies and pure macrophage colonies but not granulocyte-macrophage colonies (Figure 2C), demonstrating that this population comprises a mixture of lineage-committed GPs and MPs.
Both populations yielded similar total numbers of colonies (Figure 2D), but as expected for later stage progenitors, the GMP-Ly6C+ cells formed smaller colonies than the GMP-Ly6C− cells, resulting in a lower cell yield per progenitor (Figure 2E and data not shown). Moreover, GMP-Ly6C− cells produced colonies containing large numbers of c-Kit+ cells (progenitors) in primary cultures, whereas the GMP-Ly6C+ cultures yielded only a very small number of c-Kit+ cells (Figure 2F). Consistent with this, when total cells harvested from GMP-Ly6C− cultures were replated in secondary methylcellulose cultures they retained the capacity to form colonies, whereas replating of an equal number of cells harvested from GMP-Ly6C+ cultures yielded very few secondary colonies (Figure 2G). The inability of the GMP-Ly6C+ cells to give rise to progenitors supports the idea that they are a later progenitor stage than GMP-Ly6C− cells. Together. our data identify the GMP-Ly6C− cells as oligopotent GMPs, and the GMP-Ly6C+ cells as their lineage-committed progeny (GPs and MPs).
Hettinger et al recently reported the identification of a population of CD115-expressing monocyte progenitors in mouse bone marrow and spleen.19 Because CD115 is the receptor for the macrophage-inducing cytokine M-CSF, we wondered whether the GPs and MPs in the GMP-Ly6C+ subset could be separated on the basis of their expression of CD115. Consistent with this hypothesis, we were able to detect and isolate CD115lo and CD115hi subsets of the GMP-Ly6C+ cells (Figure 3A), and in vivo analysis showed that the GMP-Ly6C+CD115lo and GMP-Ly6C+CD115hi subsets were strongly enriched for neutrophil-producing GPs and monocyte/macrophage-producing MPs, respectively (Figure 3B-C).
CD115lo and CD115hi subsets of GMP-Ly6C+ cells are enriched for GPs and MPs, respectively. (A) CD115 expression by GMP-Ly6C− and GMP-Ly6C+ cells was assessed by flow cytometry. (B-C) One hundred thousand CD115lo and CD115hi cells isolated from the GMP-Ly6C+ subset of CD45.2 donor mice by FACS were injected IV into CD45.1 recipient mice (nonirradiated) on day 0. Spleens were harvested from recipient mice on days 3 and 4, and splenocytes were enriched for CD45.2+ (donor-derived) cells by depleting CD45.1+ cells prior to staining to detect donor cell-derived (CD45.2+) neutrophils (green gates), monocytes, and macrophages (blue gates) by flow cytometry (see supplemental Figure 4 for gating strategy). CD45.2+CD11b+ splenic cells are shown. Data presented are from 1 experiment that is representative of at least 3 independent experiments. (D) Summary of surface marker expression by oligopotent GMPs and lineage-committed GPs and MPs.
CD115lo and CD115hi subsets of GMP-Ly6C+ cells are enriched for GPs and MPs, respectively. (A) CD115 expression by GMP-Ly6C− and GMP-Ly6C+ cells was assessed by flow cytometry. (B-C) One hundred thousand CD115lo and CD115hi cells isolated from the GMP-Ly6C+ subset of CD45.2 donor mice by FACS were injected IV into CD45.1 recipient mice (nonirradiated) on day 0. Spleens were harvested from recipient mice on days 3 and 4, and splenocytes were enriched for CD45.2+ (donor-derived) cells by depleting CD45.1+ cells prior to staining to detect donor cell-derived (CD45.2+) neutrophils (green gates), monocytes, and macrophages (blue gates) by flow cytometry (see supplemental Figure 4 for gating strategy). CD45.2+CD11b+ splenic cells are shown. Data presented are from 1 experiment that is representative of at least 3 independent experiments. (D) Summary of surface marker expression by oligopotent GMPs and lineage-committed GPs and MPs.
Our ability to separate oligopotent GMPs (GMP-Ly6C−) and lineage-committed GPs (GMP-Ly6C+CD115lo) and MPs (GMP-Ly6C+CD115hi) (Figure 3D) provided us with a new opportunity to study the role of IRF8 in neutrophil vs monocyte production. Specifically, it permitted us to address whether IRF8 uses instruction and/or selection mechanisms to limit neutrophil production and promote monocyte production.
IRF8 is highly expressed by GPs and MPs, but not GMPs
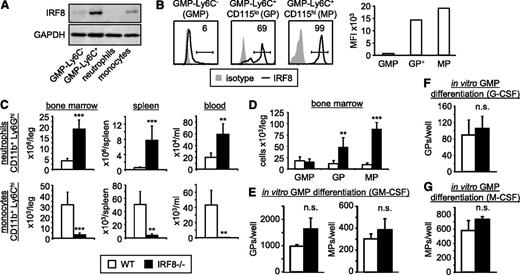
We first assessed IRF8 expression by GMPs, GPs, MPs, and differentiated cells. Analysis of the differentiated myeloid cell populations from the bone marrow revealed that Ly6Chi monocytes express IRF8, whereas neutrophils do not (Figure 4A), consistent with previous reports.8,13 Assessment of the progenitors revealed high expression of IRF8 in the GMP-Ly6C+ subset (GPs/MPs) but not the GMP-Ly6C− cells (oligopotent GMPs; Figure 4A-B). All of the GMP-Ly6C+CD115hi cells (MPs) and the majority of GMP-Ly6C+CD115lo cells (GPs) express high levels of IRF8 (Figure 4B). This suggested that IRF8 acts in the lineage-committed progenitors (GPs and MPs) rather than the oligopotent GMPs.
IRF8 is expressed by GPs and MPs but not GMPs, and is not required for GP and MP production by GMPs. (A-B) IRF8 levels in GMPs (GMP-Ly6C−), total GMP-Ly6C+, GPs (GMP-Ly6C+CD115lo), and MPs (GMP-Ly6C+CD115hi), as well as neutrophils (CD11b+Ly6Ghi Ly6Cint) and monocytes (CD11b+Ly6G−Ly6Chi) isolated from bone marrow, were assessed by western blotting using GAPDH as a loading control (A), and by intracellular flow cytometry (B). The mean fluorescence intensities (MFIs) were plotted for GMPs, IRF8+ GPs (GP+) and MPs (B, right panel). All data are representative of at least 3 independent experiments. (C-D) Neutrophils and monocytes in the bone marrow, spleen, and blood (C) and GMPs, GPs, and MPs in the bone marrow (D) of IRF8−/− mice were assessed by flow cytometry. Bone marrow numbers are per leg (femur + tibia). Data are presented as mean plus standard deviation of 5 wild-type and 5 IRF8−/− mice. Statistical significance was assessed by Student t test (**P < .01, ***P < .001). (E-G) One thousand GMPs per well were cultured in liquid media containing 20 ng/mL GM-CSF (E), 50 ng/ml G-CSF (F), or 50 ng/ml M-CSF (G), and cultures were analyzed by flow cytometry at 24 hours (F) or 48 hours (E, G) to monitor for the production of GPs and MPs (see supplemental Figure 8 for gating strategy). Two to 5 wells were pooled as necessary to permit flow cytometry analysis. Data are presented as mean plus standard deviation of at least 3 wild-type and 3 IRF8−/− mice (GM-CSF and M-CSF cultures, 5 mice per group; G-CSF cultures, 3 mice per group). Statistical significance was assessed by Student t test (**P < .01, ***P < .001). n.s., not significant.
IRF8 is expressed by GPs and MPs but not GMPs, and is not required for GP and MP production by GMPs. (A-B) IRF8 levels in GMPs (GMP-Ly6C−), total GMP-Ly6C+, GPs (GMP-Ly6C+CD115lo), and MPs (GMP-Ly6C+CD115hi), as well as neutrophils (CD11b+Ly6Ghi Ly6Cint) and monocytes (CD11b+Ly6G−Ly6Chi) isolated from bone marrow, were assessed by western blotting using GAPDH as a loading control (A), and by intracellular flow cytometry (B). The mean fluorescence intensities (MFIs) were plotted for GMPs, IRF8+ GPs (GP+) and MPs (B, right panel). All data are representative of at least 3 independent experiments. (C-D) Neutrophils and monocytes in the bone marrow, spleen, and blood (C) and GMPs, GPs, and MPs in the bone marrow (D) of IRF8−/− mice were assessed by flow cytometry. Bone marrow numbers are per leg (femur + tibia). Data are presented as mean plus standard deviation of 5 wild-type and 5 IRF8−/− mice. Statistical significance was assessed by Student t test (**P < .01, ***P < .001). (E-G) One thousand GMPs per well were cultured in liquid media containing 20 ng/mL GM-CSF (E), 50 ng/ml G-CSF (F), or 50 ng/ml M-CSF (G), and cultures were analyzed by flow cytometry at 24 hours (F) or 48 hours (E, G) to monitor for the production of GPs and MPs (see supplemental Figure 8 for gating strategy). Two to 5 wells were pooled as necessary to permit flow cytometry analysis. Data are presented as mean plus standard deviation of at least 3 wild-type and 3 IRF8−/− mice (GM-CSF and M-CSF cultures, 5 mice per group; G-CSF cultures, 3 mice per group). Statistical significance was assessed by Student t test (**P < .01, ***P < .001). n.s., not significant.
We also assessed the expression of other myeloid transcription factors in the progenitor subsets. GMPs, GPs, and MPs all express PU.1, but MPs have higher levels of PU.1 than the other progenitors (supplemental Figure 8). In contrast, c/EBPα is expressed by all GPs and MPs but only a subset of GMPs (supplemental Figure 8). These data are consistent with a requirement for both of these transcription factors in neutrophil and monocyte differentiation (c/EBPα homodimers direct neutrophil fate, whereas heterodimers with AP-1 proteins promote monocyte fate20,21 ), with higher levels of PU.1 being required for monocyte differentiation.22-24
IRF8 regulates the survival and differentiation, but not production, of GPs and MPs
To determine whether IRF8 acts in the oligopotent or lineage-committed progenitors, we next investigated the role of IRF8 in neutrophil and monocyte production using GMPs, GPs, and MPs isolated from the bone marrow of IRF8-deficient mice. Consistent with previous reports,4,25 IRF8-deficient mice had increased neutrophil numbers and very few Ly6Chi monocytes in the bone marrow, spleen, and blood (Figure 4C). Assessment of bone marrow progenitors revealed normal numbers of oligopotent GMPs in IRF8-deficient mice (Figure 4D). In vitro cultures of GMPs with GM-CSF (which supports both neutrophil and monocyte production) demonstrated that the ability of GMPs to produce GPs and MPs was not compromised by deletion of the IRF8 gene (Figure 4E; supplemental Figure 9A), consistent with the lack of IRF8 expression by the oligopotent GMPs. Moreover, wild-type and IRF8-deficient GMPs produced similar numbers of GPs in cultures supplemented with G-CSF (Figure 4F) and MPs in cultures supplemented with M-CSF (Figure 4G).
The above data indicate that IRF8 acts in the lineage-committed progenitors. Indeed, we found that GP numbers were elevated in IRF8-deficient mice (Figure 4D). Moreover, in vitro tracking of GP differentiation in cultures with G-CSF demonstrated that IRF8-deficient GPs produced more neutrophils than wild-type GPs (Figure 5A), at least in part due to a decrease in GP apoptosis (Figure 5B). These data are consistent with the increased neutrophil numbers observed in IRF8-deficient mice.
IRF8-deficient GPs yield more neutrophils and exhibit reduced apoptosis. (A) One thousand GPs (GMP-Ly6C+CD115lo) isolated from the bone marrow of wild-type or IRF8−/− mice were cultured in liquid media containing 50 ng/mL G-CSF for the indicated time points and neutrophil differentiation was monitored by flow cytometry (see supplemental Figure 2 for gating strategy). (B) GP apoptosis following 24-hour culture with G-CSF was assessed by Annexin V staining. Dotted line indicates starting GP number. All data are presented as mean plus standard deviation of independent cultures of GPs from 5 wild-type and 5 IRF8−/− mice. Statistical significance was assessed by Student t test (*P < .05, **P < .01, ***P < .001).
IRF8-deficient GPs yield more neutrophils and exhibit reduced apoptosis. (A) One thousand GPs (GMP-Ly6C+CD115lo) isolated from the bone marrow of wild-type or IRF8−/− mice were cultured in liquid media containing 50 ng/mL G-CSF for the indicated time points and neutrophil differentiation was monitored by flow cytometry (see supplemental Figure 2 for gating strategy). (B) GP apoptosis following 24-hour culture with G-CSF was assessed by Annexin V staining. Dotted line indicates starting GP number. All data are presented as mean plus standard deviation of independent cultures of GPs from 5 wild-type and 5 IRF8−/− mice. Statistical significance was assessed by Student t test (*P < .05, **P < .01, ***P < .001).
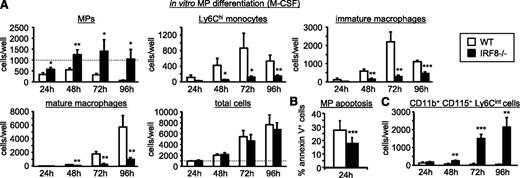
Interestingly, MP numbers were also dramatically elevated in IRF8-deficient mice (Figure 4D). This was more surprising given that these mice are defective in monocyte production. However, IRF8-deficient MPs cultured in vitro with M-CSF produced very few monocytes and macrophages (Figure 6A). Instead, MPs persisted and expanded in cultures due to reduced apoptosis, continued proliferation, and their failure to differentiate (Figure 6A-B; supplemental Figure 10). We also noticed that a population of c-Kit−CD11b+CD115+Ly6Cint cells accumulated in the IRF8-deficient MP cultures (Figure 6C; supplemental Figure 11A). Their surface marker expression indicates that they are a transitional stage between MPs and monocytes. Thus, in the absence of IRF8, some MPs begin to differentiate but are unable to form fully differentiated Ly6Chi monocytes.
IRF8-deficient MPs accumulate due to decreased apoptosis, continued proliferation, and a block in monocyte differentiation. (A-C) One thousand MPs (GMP-Ly6C+CD115hi) isolated from the bone marrow of wild-type or IRF8−/− mice were cultured in liquid media containing 50 ng/mL M-CSF for the indicated time points. Monocyte and macrophage differentiation was monitored by flow cytometry (A; see supplemental Figure 2 for gating strategy), MP apoptosis was assessed by Annexin V staining (B), and incompletely differentiated monocyte (CD11b+CD115+Ly6Cint) production was assessed by flow cytometry (C; see supplemental Figure 11A for full gating strategy). Dotted line indicates starting MP number. All data are presented as mean plus standard deviation of independent cultures of MPs from at least 5 wild-type and 5 IRF8−/− mice (A, C: 5 mice per group; B, 11 mice per group). Statistical significance was assessed by Student t test (*P < .05, **P < .01, ***P < .001).
IRF8-deficient MPs accumulate due to decreased apoptosis, continued proliferation, and a block in monocyte differentiation. (A-C) One thousand MPs (GMP-Ly6C+CD115hi) isolated from the bone marrow of wild-type or IRF8−/− mice were cultured in liquid media containing 50 ng/mL M-CSF for the indicated time points. Monocyte and macrophage differentiation was monitored by flow cytometry (A; see supplemental Figure 2 for gating strategy), MP apoptosis was assessed by Annexin V staining (B), and incompletely differentiated monocyte (CD11b+CD115+Ly6Cint) production was assessed by flow cytometry (C; see supplemental Figure 11A for full gating strategy). Dotted line indicates starting MP number. All data are presented as mean plus standard deviation of independent cultures of MPs from at least 5 wild-type and 5 IRF8−/− mice (A, C: 5 mice per group; B, 11 mice per group). Statistical significance was assessed by Student t test (*P < .05, **P < .01, ***P < .001).
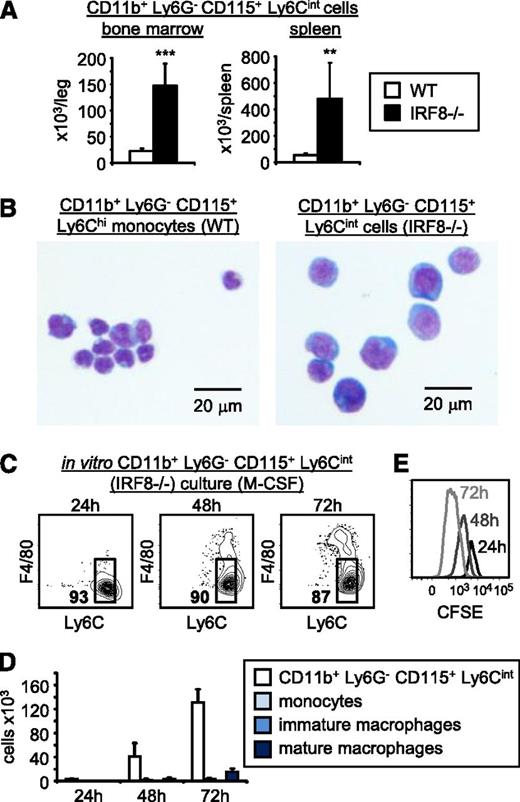
To determine whether the CD11b+CD115+Ly6Cint cells are a physiologically relevant population, we looked for these cells in the IRF8-deficient mice. Consistent with our in vitro data, we observed elevated numbers of CD11b+Ly6G−CD115+Ly6Cint cells in the bone marrow and spleen of IRF8-deficient mice (Figure 7A; supplemental Figure 11B). We confirmed that these cells are not neutrophils (they express CD115 and lack Ly6G) or macrophages (they lack F4/80) and noted that although they are CD11b+, they express lower levels of CD11b than wild-type monocytes and also lack the chemokine receptor CCR2 (supplemental Figure 11B). May-Grünwald Giemsa staining revealed that they are larger than wild-type Ly6Chi monocytes, their nuclei are less compact, and their cytosols are more basophilic and less granular (Figure 7B). Finally, we assessed their self-renewal capacity by culturing them in vitro with M-CSF for 3 days. The majority of the cells remained CD11b+Ly6G−CD115+Ly6Cint, continued to proliferate, and therefore accumulated in cultures (Figure 7C-E; supplemental Figure 12). Collectively, our data indicate that the incompletely differentiated CD11b+Ly6G−CD115+Ly6Cint cells that accumulate in IRF8−/− mice are leukemic monoblasts.
The incompletely differentiated monocytes that accumulate in IRF8-deficient mice have leukemic properties. (A) Incompletely differentiated monocytes (CD11b+Ly6G−CD115+Ly6Cint) in the bone marrow and spleens of wild-type and IRF8−/− mice were detected by flow cytometry (see supplemental Figure 11 for full gating strategy). Bone marrow numbers are per leg (femur + tibia). Data are representative of 5 mice per group. Statistical significance was assessed by Student t test (**P < .01, ***P < .001). (B) CD11b+Ly6G−CD115+Ly6Chi monocytes (wild type) and CD11b+Ly6G−CD115+Ly6Cint cells (IRF8−/−) were isolated from bone marrow and their morphology was assessed using May-Grünwald Giemsa staining. (C-E) CD11b+Ly6G−CD115+Ly6Cint cells were isolated from the bone marrow of IRF8−/− mice by FACS sorting and labeled with CFSE. One thousand cells per well were cultured in liquid media containing M-CSF for the indicated time points. (C-D) Their ability to differentiate was assessed by flow cytometry; cells presented in panel C are CD11b+. (E) CFSE dilution was measured by flow cytometry to assess proliferation of the CD11b+Ly6G−CD115+Ly6Cint cells. Three wells per time point were pooled for analysis. Data are representative of independent cultures of CD11b+Ly6G−CD115+Ly6Cint cells from 3 IRF8−/− mice.
The incompletely differentiated monocytes that accumulate in IRF8-deficient mice have leukemic properties. (A) Incompletely differentiated monocytes (CD11b+Ly6G−CD115+Ly6Cint) in the bone marrow and spleens of wild-type and IRF8−/− mice were detected by flow cytometry (see supplemental Figure 11 for full gating strategy). Bone marrow numbers are per leg (femur + tibia). Data are representative of 5 mice per group. Statistical significance was assessed by Student t test (**P < .01, ***P < .001). (B) CD11b+Ly6G−CD115+Ly6Chi monocytes (wild type) and CD11b+Ly6G−CD115+Ly6Cint cells (IRF8−/−) were isolated from bone marrow and their morphology was assessed using May-Grünwald Giemsa staining. (C-E) CD11b+Ly6G−CD115+Ly6Cint cells were isolated from the bone marrow of IRF8−/− mice by FACS sorting and labeled with CFSE. One thousand cells per well were cultured in liquid media containing M-CSF for the indicated time points. (C-D) Their ability to differentiate was assessed by flow cytometry; cells presented in panel C are CD11b+. (E) CFSE dilution was measured by flow cytometry to assess proliferation of the CD11b+Ly6G−CD115+Ly6Cint cells. Three wells per time point were pooled for analysis. Data are representative of independent cultures of CD11b+Ly6G−CD115+Ly6Cint cells from 3 IRF8−/− mice.
In conclusion, our data demonstrate that the increased GP number in the bone marrow of IRF8-deficient mice reflects greater GP survival and differentiation, whereas the increased MP number is due to elevated MP survival accompanied by continued proliferation and a block in MP differentiation, which results in accumulation of undifferentiated MPs and incompletely differentiated monocytes/leukemic monoblasts. Thus, IRF8 promotes monocyte differentiation through its action in MPs rather than by instructing MP production by GMPs, limits neutrophil production by inducing GP apoptosis, and plays a role in preventing leukemic cell production by MPs.
Discussion
We have shown that initiation of Ly6C expression marks lineage commitment in the LKS−CD34+FcγRhi fraction. Thus, Ly6C serves as a biomarker that has allowed us for the first time to separate true oligopotent GMPs (GM-CFU) from their lineage-committed progeny: GPs (G-CFU) and MPs (M-CFU). Moreover, we have provided the first strategy to isolate lineage-committed GPs (LKS−Ly6C+CD115lo). We have also shown that GMPs produce LKS−Ly6C+CD115hi progenitors that are committed to the monocytic lineage (MPs), which is consistent with a recent report that monocyte-committed progenitors express Ly6C and CD115.19 Our ability to separate GMPs, GPs, and MPs now enables us to precisely define the roles of transcription factors in the specification of myeloid cell fate.
A recent study using an IRF8-EGFP reporter mouse revealed heterogeneous IRF8 expression in the LKS−CD34+FcγRhi fraction and indicated, using in vitro analysis of colony formation by EGFPlo/int/hi subsets, that monocyte-committed progenitors (M-CFU) express higher levels of IRF8 than oligopotent GMPs (GM-CFU).18 Our data now demonstrate that IRF8 is highly expressed in lineage-committed progenitors but not oligopotent GMPs, and is not required for lineage commitment. Importantly, our observation of high IRF8 expression in both GPs and MPs demonstrates that although it is required for monocytic differentiation, its expression is not sufficient to specify monocytic fate. Moreover, previous studies reported increased numbers of myeloid colony-forming cells or LKS−CD34+FcγRhi cells in IRF8-deficient mice.1,6 We have now specifically shown that lineage-committed GPs and MPs, but not oligopotent GMPs, are elevated in the absence of IRF8.
Interestingly, although the majority of GPs express high levels of IRF8, we observed a small population of IRF8lo GPs. These could be an early stage of recently produced lineage-committed GPs. Alternatively, these GPs may have begun to progress to the next stage of differentiation by downregulating IRF8 expression. IRF8 has recently been shown to restrain neutrophil fate in monocyte/DC progenitors via inhibition of c/EBPα activity15 and may play a similar role in GPs. Thus, IRF8 downregulation may be required to permit neutrophil programming and continued differentiation.
It is likely that IRF8 primarily acts in GPs and MPs to control the neutrophil vs monocyte gene expression program, including the production of transcription factors that regulate granulopoiesis vs monopoiesis as well as proteins that functionally define the differentiated cells, for example, CD11b, Ly6C, CCR2. For instance, IRF8 has been shown to control the production of several factors that are required for monocyte differentiation, including Klf4 and Egr-1,7,25 and to act in concert with PU.1,25,26 which our data show is more highly expressed in MPs than GMPs and GPs. Moreover, Kurotaki et al recently reported that IRF8 blocks neutrophil fate in mononuclear phagocyte progenitors by physically interacting with c/EBPα to limit its binding to chromatin and thereby suppress neutrophil differentiation.15 Our data indicate that this mechanism likely also restricts the production of neutrophils by GPs, which express both IRF8 and c/EBPα.
Our data reveal an additional role for IRF8 in inducing GP and MP apoptosis to limit both granulopoietic and monopoietic differentiation. Previous reports have shown that IRF8 delivers an apoptotic signal in myeloid cells, at least in part by regulating ceramide accumulation.10,27 This mechanism is defective in CML leukemic cells that exhibit reduced IRF8 expression, rendering them resistant to apoptosis.10
Our observation that IRF8-deficient mice accumulate CD11b+Ly6G−CD115+Ly6CintCCR2− cells, which appear to represent an intermediate stage of incompletely differentiated monocytes with monoblast morphology and self-renewal properties, is consistent with monoblast/promonocyte accumulation in some AML and CML patients. It likely also explains why although some studies have demonstrated impaired Ly6Chi monocyte production by IRF8-deficient mice,25 others have reported normal numbers of monocytes defined only by CD11b and CD115 expression (CD11b+CD115+ cells comprise both incompletely differentiated and fully differentiated monocytes).1
In conclusion, our ability to separate oligopotent GMPs from their lineage-committed progeny has revealed that IRF8 regulates neutrophil and monocyte production independently in lineage-committed progenitors (GPs and MPs), rather than acting in oligopotent GMPs to instruct monocytic fate. This progenitor isolation approach will also permit future studies to precisely delineate the mechanisms that regulate myeloid cell production in the steady state, in response to infection and inflammation, and in diseases caused by defective or dysregulated myelopoiesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by funding from the Board of Governors Regenerative Medicine Institute and the Department of Biomedical Sciences at Cedars-Sinai Medical Center.
Authorship
Contribution: A.Y. and H.S.G. designed the study, analyzed data, and wrote the paper; and A.Y. performed the experiments with assistance from M.Y.N. and N.H.-K.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Helen S. Goodridge, Board of Governors Regenerative Medicine Institute, Cedars-Sinai Medical Center, 8700 Beverly Blvd, AHSP 8th Fl, Los Angeles, CA 90048; e-mail: helen.goodridge@csmc.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal