Key Points
NK cytotoxic activity limits HLH-like immunopathology in cytotoxic-deficient mice.
NK cytotoxic activity reduces T-cell activation and tissue infiltration of macrophages.
Abstract
The impairment of cytotoxic activity of lymphocytes disturbs immune surveillance and leads to the development of hemophagocytic lymphohistiocytic syndrome (HLH). Although cytotoxic T lymphocyte (CTL) control of HLH development is well documented, the role for natural killer (NK)-cell effector functions in the pathogenesis of this immune disorder remains unclear. In this study, we specifically targeted a defect in cytotoxicity to either CTL or NK cells in mice so as to dissect the contribution of these lymphocyte subsets to HLH-like disease severity after lymphocytic choriomeningitis virus (LCMV) infection. We found that NK-cell cytotoxicity was sufficient to protect mice from the fatal outcome that characterizes HLH-like disease and was also sufficient to reduce HLH-like manifestations. Mechanistically, NK-cell cytotoxicity reduced tissue infiltration by inflammatory macrophages and downmodulated LCMV-specific T-cell responses by limiting hyperactivation of CTL. Interestingly, the critical protective effect of NK cells on HLH was independent of interferon-γ secretion and changes in viral load. Therefore our findings identify a crucial role of NK-cell cytotoxicity in limiting HLH-like immunopathology, highlighting the important role of NK cytotoxic activity in immune homeostasis.
Introduction
Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells play a central role in the defense against viruses and tumors.1 CTL and NK cells perform their cytolytic function by specifically recognizing and eliminating infected and transformed cells in a process dependent on the polarized secretion of perforin and granzymes at the immunologic synapse.1,2 In humans, the importance of this effector function is highlighted by an immunopathological condition observed in patients with inborn errors affecting lymphocyte cytotoxic function.2,3 These disorders result in a life-threatening condition known as hemophagocytic lymphohistiocytic syndrome (HLH), an immune dysregulation characterized by the loss of lymphocytes homeostasis and a severe hyperinflammation.3,4 HLH patients present with nonremitting fever, hepatosplenomegaly, hypercytokinemia, accumulation of activated CD8 T cells, and organ infiltration by activated macrophages that phagocytose cellular elements and red-blood cells (ie, hemophagocytosis).4 In humans, the onset of HLH is believed to be triggered mainly by viral infection, but HLH can also occur in the absence of any detectable pathogen.3,5
Familial forms of HLH (FHL) are caused by mutations affecting the cytolytic effector protein perforin, or by proteins involved in the molecular machinery required for the biogenesis and/or transport of perforin-containing vesicles to the immune synapse.2 Thus mutations in perforin (PRF1) are responsible for FHL type 2 (FHL2),6 UNC13-D for FHL3,7 STX11 for FHL4,8 and STXBP2 for FHL5.9 HLH can also be presented with hypopigmentation as in Gricelli syndrome type 2 (RAB27A) and Chediak-Higashi syndrome (LYST).10-12
Our understanding of the immunopathological mechanisms responsible for HLH development has benefited from the availability of several murine models of HLH. Cytotoxic-deficient mice (such as Prf1, Rab27a, Unc13d, Stx11, and Lyst mice) develop a severe HLH-like syndrome after infection with lymphocytic choriomeningitis virus (LCMV).13-18 In the absence of cytotoxic activity of CD8 T cells, an accumulation of the antigen-presenting cells that continuously activate CTLs was reported in Prf1−/− mice.19 The hyperactivated CTLs secrete high levels of interferon (IFN)-γ, which appears critical for the development of HLH-like symptoms.13,20 MyD88 signaling is also thought to be important for HLH-like development, because, in Unc13d Myd88 double knockout mice, a reduction in CTL and macrophage activation has been observed.21 The crucial role of CTLs and IFN-γ for HLH-like development has been highlighted by experiments in which depletion of CTLs, or blocking of IFN-γ, prevents and reduces HLH-like manifestations, respectively.13,17,20 Despite the fact that injection of anti-NK1.1–depleting antibodies in Prf1−/− mice did not prevent development of HLH-like symptoms,13 the contribution of NK cytotoxic activity to HLH immunopathogenesis has not been fully addressed. Recently, NK cells were shown to play an important immunoregulatory role by limiting hyperactivation of CTLs in a mechanism dependent on perforin.22-24 Whether or not a similar mechanism may participate in the pathogenesis of HLH is unknown.
In this study, we dissect the specific contribution of NK-cell cytotoxic activity to HLH development. By generating murine models in which cytotoxic defects are restricted to CTL or NK cells, we show that both CTL and cytotoxic NK cells contribute to the development of HLH-like syndrome in mice after LCMV infection. Interestingly, our results show that the mechanism by which the cytotoxic function of CTL and NK cells prevents HLH-like manifestations differs. Whereas CTL cytotoxicity mainly acts on viral clearance, NK cytotoxic activity limits hyperactivation of CTL and tissue infiltration by activated macrophages. Thus our data show that these cytotoxic lymphocyte subsets play nonredundant roles in immune homeostasis and the prevention of LCMV-induced immunopathology.
Methods
Mice
Stx11flox/flox, Stx11−/−, C57BL/6J wt, and C57BL/6J-Prf1tm1Sdz/J have been described previously.16 Ncr1greenCre mice were also described previously.25 C57BL/6J Rag2−/−Il2rg−/− CD45.2 and C57BL/6J Rag2−/− CD45.1 mice were kindly provided by Dr Benedita Rocha (INEM, Paris). P14 CD45.1 mice were described previously.16 P14 Prf1−/− CD45.1 mice were generated by breeding P14 CD45.1 mice with Prf1−/− CD45.2 mice. Mice were maintained in pathogen-free conditions and were handled according to national and institutional guidelines.
Induction of HLH-like syndrome by LCMV infection
Professors Maries van de Broek and Rolf Zinkernagel (University of Zürich, Switzerland) kindly provided the WE strain of LCMV. Mice aged 8 to 14 weeks received an intraperitoneal injection of 200 plaque-forming units (PFU). Blood counts were checked after infection using an automated cell counter (MS 9-5 V; Melet Schloesing Laboratories). Serum levels of alanine and aspartate aminotransferase were determined using the VetTest Chemistry Analyzer (IDEXX Laboratories). The serum IFN-γ concentration was determined using an enzyme-linked immunosorbent assay kit (eBioscience). The serum levels of other cytokines and chemokine were quantified by cytometric bead array kit (BD Bioscience).
LCMV viral load was assessed by quantitative polymerase chain reaction as described previously.16 Briefly, cDNA was isolated from tissue samples and analyzed with primers for LCMV (forward: 5′-TCTCATCCCAACCATTTGCA-3′ and reverse: 5′-GGGAAATTTGACAGCACAACAA-3′) and β-actin (forward: 5′-CCAGCAGATGTGGATCAGCA-3′ and reverse: 5′-CTTGCGGTGCACGATGG-3′) using SYBR Green PCR Master Mix (Applied Biosystems).
In vitro CD8+ T- and NK-cell activation and degranulation assays
Spleen CD8+ T cells were purified from the spleen and activated in vitro with a T-cell activation/expansion kit (Miltenyi) in the presence of 50 U/mL of recombinant IL-2. After 5 days, T-cell degranulation was assayed by activating 4 × 105 cells with different concentrations of anti-CD3e (clone 500A2, eBioscience) in the presence of PE-coupled anti-CD107a (clone 1D4B, eBioscience) antibody. Degranulation capacity was assayed in CD8+ T cells and compared with nonactivated cells and the corresponding isotype control.
NK cells were purified from the spleen by negative selection (Miltenyi, NK isolation kit) and were cultured in vitro in the presence of 50 ng/mL IL-15 for 7 days. NK degranulation was assessed following incubation with YAC-1 target cells.
In vivo T-cell proliferation
To measure T-cell proliferation, 1 × 106 carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled perforin-sufficient or perforin-deficient P14 CD45.1 CD8 T cells were transferred intravenously into mice carrying perforin-sufficient or perforin-deficient T cells, respectively, at day −1. At day 0, recipient mice were infected intravenously with 200 PFU of LCMV. A total of 3 days later, CFSE dilution was assessed by fluorescence-activated cell sorter (FACS) analysis (gated on CD8+ CD45.1+ cells).
NK-cell depletion
Starting at day −2, anti-NK1.1–depleting antibody (clone PK136) was administered by intraperitoneal injection (50 mg) every 3 days to deplete NK1.1-expressing cells. Under these conditions, >90% of NK deletion in blood was achieved (data not shown). At day 0, mice were infected with a single dose of 200 PFU of LCMV and the HLH-like syndrome was evaluated as previously described.16
Bone marrow reconstitution
For bone marrow (BM) transfer, 3-week old Rag2−/−Il2rg−/− CD45.2 and Rag2−/− CD45.1 mice were injected intravenously with 2 × 107 marrow cells from control or Prf1−/− mice. Immune reconstitution was monitored by FACS analysis and by measuring total lymphocyte blood counts using an automated cell counter (MS 9-5 V, Melet Schloesing Laboratories). After ≥8 weeks of marrow transfer and complete immune reconstitution, mice were challenged with 200 PFU of LCMV to induce the HLH-like syndrome as described previously.
Statistical analysis
Data were analyzed with GraphPad Prism 6 software. Survival curves were analyzed using the log-rank test. All other analyses were performed using Student t tests or 1-way analysis of variance (ANOVA) with posttest. Differences were considered to be statistically significant when P < .05 (indicated as *P < .05, **P < .01, and ***P < .001).
Results
NK-cell cytotoxic activity reduces HLH-like manifestations in mice
It has been extensively documented that cytotoxicity-deficient CD8 T cells are the main driving force for immunopathogenesis in mouse models of HLH-like disease.13,17,19,26 In contrast, the contribution of NK cells to HLH-like manifestations is less clear. Depletion of NK cells in Prf1−/− mice did not prevent HLH-like development, suggesting that NK cells were dispensable for the initiation of this syndrome.13 To determine specifically the contribution of NK cells in the development of HLH-like disease, we used LCMV-infected perforin-deficient mice, as these mice develop a disease pathology that closely mimics human HLH. We generated chimeric mice by transferring BM from control or Prf1−/− mice on the CD45.2 background into Rag2−/−Il2rg−/− CD45.2 (lacking both T and NK populations) or Rag2−/− CD45.1 (lacking only T cells) mice. Following BM transfer, primary and secondary lymphoid organs were repopulated with mature T and NK cells (supplemental Figure 1A; available on the Blood Web site). When Rag2−/−Il2rg−/− mice were reconstituted with control or Prf1−/− BM as donor cells, both T and NK cells were CD45.2, that is, cytotoxic proficient (TPrf1+NKPrf1+ mice) or deficient (TPrf1-NKPrf1- mice), respectively. In contrast, when Rag2−/− CD45.1 mice were reconstituted with Prf1−/− BM, T cells were cytotoxic deficient because they developed from donor cells (CD45.2), whereas NK cells were cytotoxic proficient because they were endogenously (CD45.1) derived (TPrf1−NKPrf1+ mice) (supplemental Figure 1B).
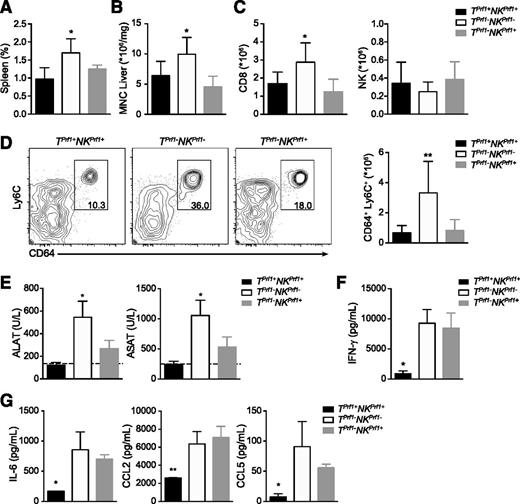
To determine the contribution of NK cytotoxic activity to HLH-like development, we infected TPrf1+NKPrf1+, TPrf1−NKPrf1−, and TPrf1−NKPrf1+ mice with a single dose of 200 PFU of LCMV. As expected, on LCMV injection, TPrf1+NKPrf1+ mice survived the infection, whereas mice, in which both T and NK cells were deficient in cytotoxic activity, succumbed before 28 days postinfection (Figure 1A). In addition to the increased susceptibility to LCMV, TPrf1−NKPrf1− mice presented a significant loss of body weight and a dramatic drop in body temperature compared with TPrf1+NKPrf1+ mice (Figure 1A). Interestingly, the presence of cytotoxic NK cells in an otherwise perforin-deficient recipient (TPrf1−NKPrf1+ mice) could significantly prolong survival after infection (Figure 1A). TPrf1−NKPrf1+ mice presented with an intermediate phenotype in terms of body weight and body temperature. Compared with control mice, TPrf1−NKPrf1− mice presented the typical biological features of HLH-like immunopathology, including leukopenia, decreased numbers of red blood cells, hematocrit and platelets, and lack of neutrophilia on day 14 postinfection (Figure 1B-D). In contrast, TPrf1−NKPrf1+ mice displayed normal RBC and platelet counts on day 14 postinfection (Figure 1C), although blood leukocyte numbers were reduced compared with TPrf1+NKPrf1+ mice (Figure 1B,D).
Presence of NK-cell cytotoxic activity reduces HLH-like manifestations in mice.Rag2−/−Il2rg−/− and Rag2−/− mice were reconstituted with BM from Prf1−/− and control B6 donors. After 8 weeks, TPrf1−NKPrf1− (open bars/open squares), TPrf1−NKPrf1+ (gray bars/gray circles), and TPrf1+NKPrf1+ mice (black bars/black squares) were infected with 200 PFU of LCMV-WE. Clinical and biochemical parameters were determined after infection. (A) Survival, body weight, and body temperature. (B) White blood cell counts were analyzed on day 14 postinfection. (C) Red blood cell, hematocrit, and platelets counts on day 14 postinfection. (D) The time course of neutrophils counts. Data (mean ± standard error of the mean [SEM]) are representative of 3 to 4 independent experiments with at least 3 mice in each group. For survival experiments TPrf1−NKPrf1− (n = 10), TPrf1−NKPrf1+ (n = 9), and TPrf1+NKPrf1+ (n = 12) mice. Dotted lines represent minimal normal values for control mice. Statistical analysis was performed using log-rank test or 1-way ANOVA. **P < .01; ***P < .001.
Presence of NK-cell cytotoxic activity reduces HLH-like manifestations in mice.Rag2−/−Il2rg−/− and Rag2−/− mice were reconstituted with BM from Prf1−/− and control B6 donors. After 8 weeks, TPrf1−NKPrf1− (open bars/open squares), TPrf1−NKPrf1+ (gray bars/gray circles), and TPrf1+NKPrf1+ mice (black bars/black squares) were infected with 200 PFU of LCMV-WE. Clinical and biochemical parameters were determined after infection. (A) Survival, body weight, and body temperature. (B) White blood cell counts were analyzed on day 14 postinfection. (C) Red blood cell, hematocrit, and platelets counts on day 14 postinfection. (D) The time course of neutrophils counts. Data (mean ± standard error of the mean [SEM]) are representative of 3 to 4 independent experiments with at least 3 mice in each group. For survival experiments TPrf1−NKPrf1− (n = 10), TPrf1−NKPrf1+ (n = 9), and TPrf1+NKPrf1+ (n = 12) mice. Dotted lines represent minimal normal values for control mice. Statistical analysis was performed using log-rank test or 1-way ANOVA. **P < .01; ***P < .001.
An HLH-like syndrome in TPrf1−NKPrf1− mice was associated with splenomegaly and liver infiltration by activated immune cells (Figure 2A-B). Interestingly, splenomegaly and liver infiltration after LCMV infection was comparable between TPrf1+NKPrf1+ and TPrf1−NKPrf1+ mice (Figure 2A-B). MNC liver infiltrate in TPrf1−NKPrf1− mice was highly enriched in activated CD8 T cells, whereas percentage and absolute numbers of these cells were lower in TPrf1+NKPrf1+ and TPrf1−NKPrf1+ mice (Figure 2C). Strikingly, similar absolute numbers of liver-infiltrating NK cells were detected in all 3 conditions, indicating that phenotypic differences between TPrf1−NKPrf1− and TPrf1−NKPrf1+ mice were qualitative (Figure 2C). Tissue-infiltration of macrophages is one of the hallmarks of HLH, and macrophages are among the most important effector populations in the later phases of HLH immunopathology. Interestingly, macrophage liver infiltration on day 14 postinfection was significantly reduced in TPrf1+NKPrf1+ and TPrf1−NKPrf1+ mice, as compared with fully cytotoxic-deficient mice (Figure 2D). Reduction of inflammatory macrophages (defined as CD64+ Ly6C+ cells)27,28 infiltration in TPrf1−NKPrf1+ mice was observed both in terms of percentage and absolute numbers compared with TPrf1−NKPrf1− mice (Figure 2D). Interestingly, reduced numbers of inflammatory macrophages correlated with a reduction in levels of serum liver transaminases (alanine aminotransferase and aspartate aminotransferase) in TPrf1+NKPrf1+ and TPrf1−NKPrf1+ mice compared with mice that lacked all cytotoxic activity (Figure 2E).
Presence of NK-cell cytotoxic activity reduces biological features of HLH-like syndrome.Rag2−/−Il2rg−/− and Rag2−/− mice were reconstituted with BM from Prf1−/− and control B6 donors. After 8 weeks, TPrf1−NKPrf1− (open bars), TPrf1−NKPrf1+ (gray bars), and TPrf1+NKPrf1+ mice (black bars) were infected with 200 PFU of LCMV-WE. (A) Spleen size expressed as percentage of body weight on day 14 postinfection. (B) Mononuclear cell (MNC) infiltration in liver on day 14 postinfection. (C) Absolute numbers of CD8+ and NK1.1+ in liver. (D) FACS analysis of MNC from liver gated on CD3− CD19− NK1.1− (left panel). Quantification of absolute numbers of CD64+ Ly6C+ in liver on day 14 postinfection. (E) Serum alanine and aspartate aminotransferase (alanine aminotransferase and aspartate aminotransferase, respectively) levels in infected mice were analyzed on day 14 postinfection. (F) Serum IFN-γ on day 8 postinfection. (G) Serum IL-6 (left panel), CCL2 (middle panel), and CCL5 levels (right panel) on day 14 postinfection. Data (mean ± SEM) are representative of 3 to 4 independent experiments with at least 3 mice in each group. Dotted lines represent minimal normal values for control mice. Statistical analysis was performed using 1-way ANOVA or Student t test. *P < .05; **P < .01.
Presence of NK-cell cytotoxic activity reduces biological features of HLH-like syndrome.Rag2−/−Il2rg−/− and Rag2−/− mice were reconstituted with BM from Prf1−/− and control B6 donors. After 8 weeks, TPrf1−NKPrf1− (open bars), TPrf1−NKPrf1+ (gray bars), and TPrf1+NKPrf1+ mice (black bars) were infected with 200 PFU of LCMV-WE. (A) Spleen size expressed as percentage of body weight on day 14 postinfection. (B) Mononuclear cell (MNC) infiltration in liver on day 14 postinfection. (C) Absolute numbers of CD8+ and NK1.1+ in liver. (D) FACS analysis of MNC from liver gated on CD3− CD19− NK1.1− (left panel). Quantification of absolute numbers of CD64+ Ly6C+ in liver on day 14 postinfection. (E) Serum alanine and aspartate aminotransferase (alanine aminotransferase and aspartate aminotransferase, respectively) levels in infected mice were analyzed on day 14 postinfection. (F) Serum IFN-γ on day 8 postinfection. (G) Serum IL-6 (left panel), CCL2 (middle panel), and CCL5 levels (right panel) on day 14 postinfection. Data (mean ± SEM) are representative of 3 to 4 independent experiments with at least 3 mice in each group. Dotted lines represent minimal normal values for control mice. Statistical analysis was performed using 1-way ANOVA or Student t test. *P < .05; **P < .01.
IFN-γ is known to be a critical factor in LCMV-induced HLH-like syndrome, because in vivo neutralization of IFN-γ can reduce HLH-like manifestations in mice.13,20 To determine whether or not the moderate HLH-like manifestations observed in TPrf1−NKPrf1+ mice correlated with reduced levels of IFN-γ secretion, we measured IFN-γ production on day 8 postinfection. As expected, TPrf1−NKPrf1− mice had higher levels of IFN-γ compared with TPrf1+NKPrf1+ mice (Figure 2F). Interestingly, TPrf1−NKPrf1+ mice displayed serum IFN-γ levels similar to TPrf1−NKPrf1− mice (Figure 2F), suggesting that reduction of HLH-like manifestations in the presence of cytotoxic-proficient NK cells was not mediated by a reduction in serum IFN-γ levels. HLH-like syndrome is characterized by production of high levels of inflammatory cytokines that contribute to the hyperinflammatory environment. Interestingly, serum levels of inflammatory cytokines and chemokines, including IL-6, CCL2, and CCL5, were similar in TPrf1−NKPrf1− and TPrf−NKPrf+ mice (Figure 2G).
Overall, these data show that when cytotoxic potential in mice is strictly limited to NK cells, HLH-like disease manifestations triggered by LCMV infection are dramatically reduced compared with mice that globally lack cytotoxic activity.
Reduction of HLH-like manifestations in T−NK+ mice is independent of viral load
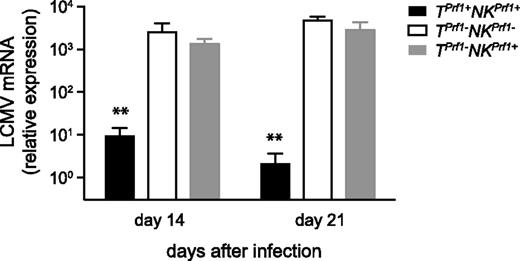
Defective viral control and persistent LCMV infection are considered key factors in HLH-like pathogenesis. To address whether the reduction of HLH-like manifestations in mice carrying cytotoxic-proficient NK cells was accompanied by a more efficient viral clearance, we measured viral load in the liver 14 and 21 days on infection in TPrf1+NKPrf1+, TPrf1−NKPrf1−, and TPrf1−NKPrf1+ mice. Cytotoxic-proficient TPrf1+NKPrf1+ mice were able to control LCMV replication and successfully cleared the virus (Figure 3). In contrast, cytotoxic-deficient TPrf1−NKPrf1− mice were unable to control the virus and displayed a high LCMV load (Figure 3). Interestingly, TPrf1−NKPrf1+ mice were unable to control LCMV replication and showed similar viral titers as compared with mice that were completely deficient in cytotoxicity (Figure 3). Similar results were observed in the spleen 14 days after LCMV infection (supplemental Figure 2). These data thus show that the improvement of HLH-like manifestations associated with cytotoxic-proficient NK cells appears to be independent of viral replication control in vivo.
Defective control of LCMV infection in NK cytotoxic-deficient mice.Rag2−/−Il2rg−/− and Rag2−/− mice were reconstituted with BM from Prf1−/− and control B6 donors. TPrf1−NKPrf1− (open bars), TPrf1−NKPrf1+ (gray bars), and TPrf1+NKPrf1+ mice (black bars) were infected with 200 PFU of LCMV-WE. A total of 14 days after infection, LCMV titers in the liver were determined. Data (mean ± SEM) are representative of 3 independent experiments. Statistical analysis was performed using Student t test. **P < .01.
Defective control of LCMV infection in NK cytotoxic-deficient mice.Rag2−/−Il2rg−/− and Rag2−/− mice were reconstituted with BM from Prf1−/− and control B6 donors. TPrf1−NKPrf1− (open bars), TPrf1−NKPrf1+ (gray bars), and TPrf1+NKPrf1+ mice (black bars) were infected with 200 PFU of LCMV-WE. A total of 14 days after infection, LCMV titers in the liver were determined. Data (mean ± SEM) are representative of 3 independent experiments. Statistical analysis was performed using Student t test. **P < .01.
In vivo depletion of cytotoxic-proficient NK cells restores HLH-like syndrome severity
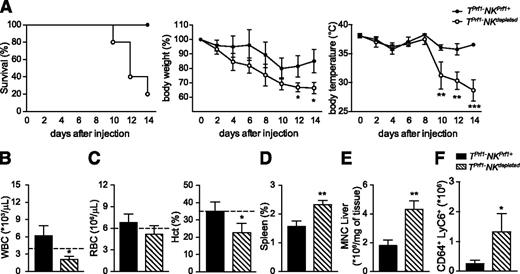
To determine whether the improvement of HLH-like manifestations observed in TPrf1−NKPrf1+ mice was dependent on NK cells and not on the presence of other innate lymphocyte populations present in Rag2−/− mice, we compared HLH-like manifestations of LCMV infected TPrf1−NKPrf1+ mice after NK-cell depletion. Mice were injected with an anti-NK1.1–depleting antibody every third day, starting 2 days before infection. As previously shown, on infection, all TPrf1−NKPrf1+ mice survived, whereas TPrf1−NKdepleted mice were highly susceptible to LCMV infection (Figure 4A). In parallel, NK depletion in TPrf1−NKPrf1+ mice led to major loss of body weight and body temperature (Figure 4A) similar to the ones observed in fully cytotoxic-deficient mice (Figure 1). Depletion of endogenous NK cells in TPrf1−NKPrf1+ mice also had an impact on other HLH-like manifestations. At 14 days postinfection, TPrf1−NKdepleted mice presented with leukopenia, anemia, splenomegaly, and organ infiltration by total leukocytes and activated macrophages (Figure 4B-F), similar to mice in which both CTL- and NK-cell populations were cytotoxic deficient (Figures 1 and 2). Thus these data show that the improvement of HLH-like manifestations observed in TPrf1−NKPrf1+ mice depend on the presence of endogenous cytotoxic-proficient NK cells. In this setting, NK cytotoxic activity appears to be sufficient to prevent partially the HLH-like development in mice.
Improvement in HLH-like manifestations in Prf1-reconstituted Rag2−/− mice depends on NK cells.Rag2−/− mice were reconstituted with BM from Prf1−/− donors. After 8 weeks, TPrf1−NKPrf1+ (black bars/black circles) and TPrf1−NKPrf1+ injected with anti-NK1.1–depleting antibody (dashed bars/open circles) were infected with 200 PFU of LCMV-WE. Clinical and biochemical parameters were determined after infection. (A) Survival, body weight, and body temperature. (B) White blood cell counts were analyzed on day 14 postinfection. (C) Red blood cell and hematocrit counts on day 14 postinfection. (D) Spleen size expressed as percentage of body weight on day 14 postinfection. (E) MNC infiltration in liver on day 14 postinfection. (F) Quantification of absolute numbers of CD64+ Ly6C+ in liver on day 14 postinfection. Data (mean ± SEM) are representative of 2 independent experiments with at least 3 mice in each group. Statistical analysis was performed using Student t test. Dotted lines represent minimal normal values for control mice. *P < .05; **P < .01.
Improvement in HLH-like manifestations in Prf1-reconstituted Rag2−/− mice depends on NK cells.Rag2−/− mice were reconstituted with BM from Prf1−/− donors. After 8 weeks, TPrf1−NKPrf1+ (black bars/black circles) and TPrf1−NKPrf1+ injected with anti-NK1.1–depleting antibody (dashed bars/open circles) were infected with 200 PFU of LCMV-WE. Clinical and biochemical parameters were determined after infection. (A) Survival, body weight, and body temperature. (B) White blood cell counts were analyzed on day 14 postinfection. (C) Red blood cell and hematocrit counts on day 14 postinfection. (D) Spleen size expressed as percentage of body weight on day 14 postinfection. (E) MNC infiltration in liver on day 14 postinfection. (F) Quantification of absolute numbers of CD64+ Ly6C+ in liver on day 14 postinfection. Data (mean ± SEM) are representative of 2 independent experiments with at least 3 mice in each group. Statistical analysis was performed using Student t test. Dotted lines represent minimal normal values for control mice. *P < .05; **P < .01.
NK-cell cytotoxic defect does not induce HLH-like syndrome in mice but causes leukocytosis
These results suggest that NK cytotoxic activity contributes to prevent HLH-like development by a mechanism independent of viral clearance. To determine whether defective NK cytotoxic activity was sufficient to induce HLH-like development upon viral infection, we studied a second mouse model in which a defect in cytotoxicity was restricted to NK cells. As mice harboring a conditional perforin allele are not yet available, we created mice with a NK-cell–specific ablation of Stx11, a protein that is required for the release of perforin-containing cytotoxic granules.16,17,29 We crossed loxP-flanked Stx11 mice16 with a transgenic mouse line expressing Cre recombinase under the promoter of NKp46 (Ncr1)25 that allows for specific ablation of Stx11 expression in NK cells (T+NKStx11− mice). As control, we used littermate Ncr1cre-Stx11+/+ mice. T+NKStx11− mice developed normally, were fertile, and showed no variations in the percentage of lymphoid and myeloid cell populations compared with littermate controls (data not shown). As expected, NK cells from T+NKStx11− mice failed to degranulate in vitro, whereas CD8 T cells degranulated normally on CD3/CD28 activation as assessed by CD107 expression (supplemental Figure 3).
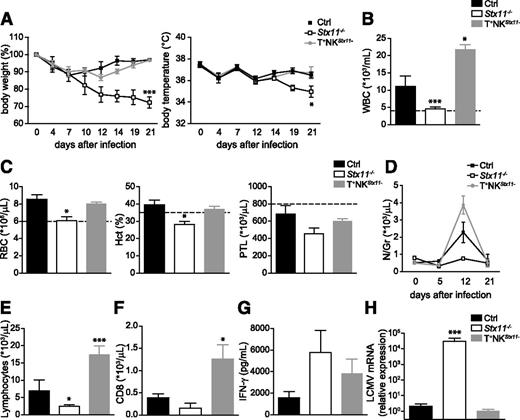
To determine whether defective NK-cell cytotoxic activity was sufficient to induce HLH-like development, control, Stx11−/− and T+NKStx11− mice were injected with a single dose of LCMV (200 PFU). Although control mice restrain LCMV infection successfully, Stx11-deficient mice developed most clinical and biological features of HLH-like syndrome including loss of body weight (Figure 5A), drop in body temperature (Figure 5A), hunched posture, lethargy, pancytopenia (Figure 5B-C), and lack of neutrophilia (Figure 5D). In contrast to complete Stx11-deficient mice, T+NKStx11− mice did not develop HLH-like manifestations and were similar to control mice in terms of body weight, temperature, RBC and platelet counts, and hematocrit (Figures 5A,C).
Defects of NK cytotoxic activity are not sufficient to induce HLH-like development in mice.Stx11−/− (open bars/open squares), T+NKStx11− (gray bars/gray circles), and control (black bars/black squares) mice were infected with 200 PFU of LCMV-WE. Clinical and biochemical parameters were determined after infection. (A) Body weight and body temperature. (B) White blood cell counts were analyzed on day 12 postinfection. (C) Red blood cell, hematocrit, and platelets counts on day 12 postinfection. (D) The time course of neutrophils counts. (E) Lymphocytes counts were analyzed on day 12 postinfection. (F) Absolute numbers of CD8 lymphocytes in blood on day 12 postinfection. (G) Serum IFN-γ on day 8 postinfection. (H) LCMV titers in liver on day 21-postinfection. Data (mean ± SEM) are representative of 2 independent experiments with at least 3 mice in each group. Dotted lines represent minimal normal values for control mice. Statistical analysis was performed using 1-way ANOVA or Student t test. *P < .05; ***P < .001.
Defects of NK cytotoxic activity are not sufficient to induce HLH-like development in mice.Stx11−/− (open bars/open squares), T+NKStx11− (gray bars/gray circles), and control (black bars/black squares) mice were infected with 200 PFU of LCMV-WE. Clinical and biochemical parameters were determined after infection. (A) Body weight and body temperature. (B) White blood cell counts were analyzed on day 12 postinfection. (C) Red blood cell, hematocrit, and platelets counts on day 12 postinfection. (D) The time course of neutrophils counts. (E) Lymphocytes counts were analyzed on day 12 postinfection. (F) Absolute numbers of CD8 lymphocytes in blood on day 12 postinfection. (G) Serum IFN-γ on day 8 postinfection. (H) LCMV titers in liver on day 21-postinfection. Data (mean ± SEM) are representative of 2 independent experiments with at least 3 mice in each group. Dotted lines represent minimal normal values for control mice. Statistical analysis was performed using 1-way ANOVA or Student t test. *P < .05; ***P < .001.
Whereas complete Stx11-deficient mice developed a severe leukopenia and neutropenia on LCMV infection, T+NKStx11− mice presented a significant leukocytosis, even compared with control mice (Figure 5B,D). T+NKStx11− mice had higher numbers of neutrophils and CD4 and CD8 lymphocytes in the blood at 12 days postinfection (Figure 5D-F and data not shown). T+NKStx11− mice presented intermediate levels of serum IFN-γ compared with control and complete Stx11-deficient mice (Figure 5G). Interestingly, in accordance with the lack of HLH-like manifestations, T+NKStx11− mice completely eliminated the virus 21 days after infection, suggesting that CD8 T-cell cytotoxic activity was sufficient to control LCMV (Figure 5H).
These data show that the restricted defect of cytotoxic activity in NK cells was not sufficient to induce HLH-like immunopathology in mice, as far as T+NKStx11− mice were able to control LCMV infection. Nonetheless, the absence of cytotoxic function in NK cells is correlated with an exacerbated leukocytosis and a limited secretion of IFN-γ, suggesting a regulatory role for NK cells in lymphocyte proliferation and in mounting a physiologic response against LCMV infection.
NK-cell cytotoxic activity controls T-cell response in vivo
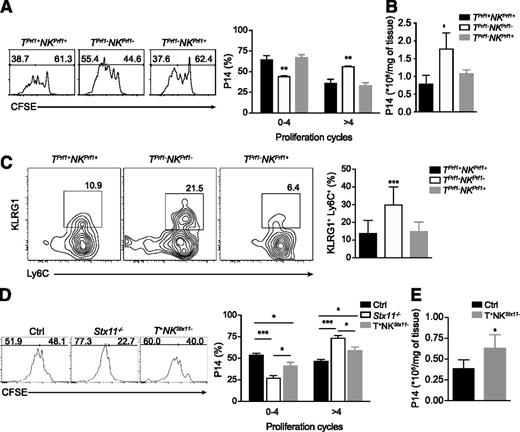
Taken together, the beneficial role of NK-cell cytotoxic activity in alleviating HLH-like manifestations as observed in TPrf1−NKPrf1+ mice (Figure 1) and the differential immune response observed in LCMV-infected T+NKStx11− mice (Figure 5) suggest that NK cytotoxic activity modulates CTL proliferation on LCMV viral infection. To test this hypothesis, we assessed CD8 T-cell proliferation in TPrf1−NKPrf1+ and T+NKStx11− mice and compared them with their respective controls. We adoptively transferred perforin-sufficient or perforin-deficient CFSE-labeled P14 T-cell receptor transgenic CD8 T cells into TPrf1+NKPrf1+, TPrf1−NKPrf1− and TPrf1−NKPrf1+ mice, which were subsequently infected with 200 PFU LCMV. A total of 3 days after infection, CFSE dilution was analyzed as a measure of CTL proliferation. Although LCMV titers were similar in all conditions (data not shown), P14 CD8 T cells exhibited a higher proliferative capacity in TPrf1−NKPrf1− mice as compared to TPrf1+NKPrf1+ mice (Figure 6A). In TPrf1−NKPrf1+ mice, P14 CD8 proliferation was similar to the level observed in TPrf1+NKPrf1+ mice (Figure 6A). Accordingly, absolute numbers of P14 CD8 cells were significantly higher in the spleen of complete cytotoxic-deficient mice compared with control TPrf1+NKPrf1+ mice (Figure 6B). Strikingly, the presence of NK cytotoxic-proficient cells reduced absolute numbers of P14 CD8 T cells in the spleen to values similar to those seen in TPrf1+NKPrf1+ mice (Figure 6B). Interestingly, during the course of the anti-LCMV response, activation of endogenous CD8 T cells was also reduced in TPrf1−NKPrf1+ mice compared with fully cytotoxic-deficient mice. The fraction of KLRG1+ Ly6C+ among endogenous CD8 T cells was increased twofold in TPrf1−NKPrf1− mice compared with TPrf1+NKPrf1+ and TPrf1−NKPrf1+ mice (Figure 6C).
NK cytotoxic activity controls T-cell activation. (A) Rag2−/−Il2rg−/− and Rag2−/− mice were reconstituted with BM from Prf1−/− and control B6 donors. After 8 weeks, reconstituted mice were adoptively transferred with 1 × 106 CFSE labeled perforin-sufficient or perforin-deficient P14 cells. The day after, mice were infected with 200 PFU of LCMV-WE. The figure shows representative histograms of CFSE staining in P14 cells 3 days after infection transferred into TPrf1+NKPrf1+ (left panel/black bars), TPrf1−NKPrf1− (middle panel/open bars) and TPrf1−NKPrf1+ (right panel/gray bars). Graph represents the mean ± SEM values from 2 independent experiments with 3 mice in each group. (B) Absolute numbers of P14 cells per spleen 3 days after infection. Data (mean ± SEM) are representative of 2 independent experiments with 3 mice in each group. (C) TPrf1−NKPrf1− (open bars/middle panel), TPrf1−NKPrf1+ (gray bars/right panel), and TPrf1+NKPrf1+ mice (black bars/left panel) were infected with 200 PFU of LCMV-WE. The figure shows representative FACS analysis of blood MNC gated on CD8+. Graph represents the quantification of KLRG1+ Ly6C+ CD8+ cells from blood on day 8 postinfection. Data (mean ± SEM) are representative of 3 to 4 independent experiments. (D) Control (left panel/black bars), Stx11−/− (middle panel/open bars), and T+NKStx11− (right panel/gray bars) mice were adoptively transferred with 1 × 106 CFSE-labeled perforin-sufficient or perforin-deficient P14 cells. The day after, mice were infected with 200 PFU of LCMV-WE. The figure shows representative histograms of CFSE staining in P14 cells 3 days after infection and the percentages of cells dividing between 0 and 4 times and more than 4 times. Graph represents the mean ± SEM values from 2 independent experiments with 3 mice in each group. (E) Absolute numbers of P14 cells per spleen 3 days after infection in control (black bars) and T+NKStx11− (gray bars) mice. Data (mean ± SEM) are representative of 2 independent experiments with 3 mice in each group. Statistical analysis was performed using 1-way ANOVA or Student t-test. *P < .05; **P < .01; ***P < .001.
NK cytotoxic activity controls T-cell activation. (A) Rag2−/−Il2rg−/− and Rag2−/− mice were reconstituted with BM from Prf1−/− and control B6 donors. After 8 weeks, reconstituted mice were adoptively transferred with 1 × 106 CFSE labeled perforin-sufficient or perforin-deficient P14 cells. The day after, mice were infected with 200 PFU of LCMV-WE. The figure shows representative histograms of CFSE staining in P14 cells 3 days after infection transferred into TPrf1+NKPrf1+ (left panel/black bars), TPrf1−NKPrf1− (middle panel/open bars) and TPrf1−NKPrf1+ (right panel/gray bars). Graph represents the mean ± SEM values from 2 independent experiments with 3 mice in each group. (B) Absolute numbers of P14 cells per spleen 3 days after infection. Data (mean ± SEM) are representative of 2 independent experiments with 3 mice in each group. (C) TPrf1−NKPrf1− (open bars/middle panel), TPrf1−NKPrf1+ (gray bars/right panel), and TPrf1+NKPrf1+ mice (black bars/left panel) were infected with 200 PFU of LCMV-WE. The figure shows representative FACS analysis of blood MNC gated on CD8+. Graph represents the quantification of KLRG1+ Ly6C+ CD8+ cells from blood on day 8 postinfection. Data (mean ± SEM) are representative of 3 to 4 independent experiments. (D) Control (left panel/black bars), Stx11−/− (middle panel/open bars), and T+NKStx11− (right panel/gray bars) mice were adoptively transferred with 1 × 106 CFSE-labeled perforin-sufficient or perforin-deficient P14 cells. The day after, mice were infected with 200 PFU of LCMV-WE. The figure shows representative histograms of CFSE staining in P14 cells 3 days after infection and the percentages of cells dividing between 0 and 4 times and more than 4 times. Graph represents the mean ± SEM values from 2 independent experiments with 3 mice in each group. (E) Absolute numbers of P14 cells per spleen 3 days after infection in control (black bars) and T+NKStx11− (gray bars) mice. Data (mean ± SEM) are representative of 2 independent experiments with 3 mice in each group. Statistical analysis was performed using 1-way ANOVA or Student t-test. *P < .05; **P < .01; ***P < .001.
To determine the impact of defective NK cytotoxic activity on CTL activation, we adoptively transferred perforin-sufficient or perforin-deficient P14 cells to control, Stx11−/− and T+NKStx11− mice, and, 3 days after LCMV infection, P14 cell proliferation was assessed. P14 cell proliferation was found significantly higher in fully cytotoxic-deficient mice compared with control mice in terms of P14 cells, presenting a higher percentage of cells in proliferation and total number of P14 cells extant in the spleen (Figure 6D). Notably, in T+NKStx11− mice P14 CD8 T cells proliferated significantly more compared to control mice, as a higher percentage of P14 cells presented an increased number of division cycles (Figure 6D). The absolute counts of P14 cells per spleen were moderately increased in T+NKStx11− mice as compared with those measured in the spleen of control mice (Figure 6E). Altogether, these results show that NK-cell cytotoxic activity controls activation of cytotoxic CD8 T cells, suggesting that in a cytotoxic-deficient host, the loss of such a mechanism could contribute to the hyperactivation of lymphocytes in the settings of LCMV infection, which is a hallmark of the immunopathological events responsible for HLH-like development.
Discussion
In the current study, we dissected the specific roles for cytotoxic NK and T cells to HLH-like development, by targeting the cytotoxic defect to each of the 2 main cytotoxic lymphocyte populations. Firstly, we found that in mice, CD8 T cells with impaired cytotoxicity (perforin-deficient CTLs) are not sufficient to induce the full clinical picture of HLH-like syndrome. HLH-like syndrome with all clinical signs only develops when also NK cells show defective cytotoxicity. In the case where T cells but not NK cells displayed impaired cytotoxic activity, LCMV-infected mice developed reduced immunopathology and milder clinical manifestations of HLH-like syndrome, and, more importantly survived LCMV challenge that was lethal for mice harboring noncytotoxic lymphocytes. Secondly, we demonstrated that defective NK cytotoxic activity was not sufficient to induce HLH-like syndrome but was nevertheless associated with transient lymphocytosis. During the acute phase of the antiviral response against LCMV infection, we observed an exacerbated and transient expansion of lymphocytes, culminating to a robust immune response and a successful viral clearance in conditional NK cytotoxic-deficient mice. Other reports have shown similar results in models of acute LCMV viral infection, where depletion or absence of NK cells favors a stronger CD8 T-cell response and more efficient viral clearance.22,23 Altogether, our study emphasizes the critical immunoregulatory role of NK-cell cytotoxicity during antiviral immune response and HLH-like development, adding thus a new layer of complexity regarding the contribution of perforin-dependent mechanisms to the regulation of immune homeostasis.
NK cells control the immune response by secreting cytokines, controlling antigen presentation, and directly killing infected or tumor-transformed cells.30 There is growing evidence that NK cells control CD8 T-cell response via a perforin-dependent mechanism by limiting CD4 help during the early stages of CTL activation and differentiation and/or by direct killing of early-activated CTLs.22,23 Several NK receptors have been shown to participate in this process, especially NKG2D and NKp46.23,31 It has been shown that during the early stages of anti-LMCV response, CTLs can escape NK surveillance by increasing the expression of major histocompatibility complex-I inhibitory NK ligands and by decreasing the expression of activating NK-receptor ligands in a type I IFN-dependent process.31,32 In our study, by using either BM reconstituted or conditional knockout mice, we show that (1) the presence of NK-cytotoxic–proficient cells restrains the accumulation of LCMV-specific CD8 T cells, and (2) that defective NK-cell cytotoxicity correlates with a higher proliferation rate of antigen-specific CTLs. Whether NK cells modulate CD8 T-cell responses by direct or indirect NK-cell/T-cell recognition and the specific receptors involved in this process is a matter of further investigation.
Our study demonstrates that NK-cell cytotoxic activity is essential for preventing HLH-like development caused by LCMV infection in mice. We speculate that, because of this activity, NK cells can directly limit CTL hyperactivation and/or function. Another possibility is that NK cells also interfere with the effector phase of HLH-like syndrome by targeting activated macrophages and therefore limiting tissue destruction. Importantly, the diminished immunopathology observed in TPrf1−NKPrf1+ mice supports both hypotheses as far as it correlated with a reduction in CTL activation and with decreased inflammatory macrophage infiltration in the tissues of infected mice. Notably, our data confirmed previous observation regarding the role of NK cytotoxicity in reducing CTL activation in a disease-relevant context.
Interestingly, the reduction in HLH-like manifestations observed in the presence of NK cytotoxic activity was independent of IFN-γ levels and viral load after infection. The persistent viral infection observed in mice carrying cytotoxic-proficient NK cells underlines the prominent role of CD8 T cells in LCMV clearance. Indeed, defective NK cytotoxic activity did not prevent normal LCMV clearance. This emphasizes the concept that in cytotoxic-deficient mice, viral infection is required to trigger HLH-like syndrome but does not correlate with the course of the disease.
We and others have shown the principal role of IFN-γ in HLH-like development caused by LCMV.13,20 Strikingly, the beneficial effect of NK cytotoxic activity in reducing HLH-like pathology was not related to a decrease in IFN-γ serum levels in TPrf1−NKPrf1+ mice, as would have been expected. However, IFN-γ levels were measured in the serum and this does not necessarily reflect the actual concentration in the tissue. The cellular source of IFN-γ in the tissues during HLH-like remains a question to be addressed. Furthermore, the sustained secretion of inflammatory cytokines observed in NK-proficient mice suggests that NK cytotoxicity rather interferes directly with effector cells that could be T cells and/or macrophages.
HLH is a heterogeneous syndrome that can present variations in the manifestations and symptoms observed in patients.3 Some of these variations can be attributed to the nature of the genetic cause, environmental factors, etc. Whether some of these variations can be attributed to residual cytotoxic activity in 1 of the 2 main lymphocyte populations might be an appealing possibility. The fact that residual NK cytotoxic activity can improve HLH-like manifestations puts forward alternative therapeutic options that include the stimulation of complement-dependent cytotoxic mechanisms to eliminate hyperactivated CD8 T cells by the administration of Fc-fusion(s) proteins targeting the receptors/ligands involved in this cytotoxic process, as has been proposed in an experimental tumor model.33
In this report we have highlighted a previously unappreciated role of NK cytotoxicity in immune homeostasis by dissecting the specific contribution of NK and CD8 T cells to HLH-like development, suggesting that cytotoxic lymphocytes (both CTL and NK cells) regulate the final outcome of the immune response and the balance between physiological and pathological conditions by a variety of additive and complex cellular mechanisms beyond viral clearance.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Corina Dragu and Imagine Institute’s animal facility for their assistance.
This work was supported by fellowships from the Association pour la Recherche sur le Cancer and Becas Chile (F.E.S.) and by fellowships from the Association pour la Recherche sur le Cancer and the Imagine Foundation (S.M.). This work was also supported by the French National Institutes of Health and Medical Research (INSERM), the Agence National de la Recherche (HLH-Cytotox/ANR-12-BSV1-0020-01), the ARC Foundation (grant PJA 2013120047), the European Research Council (PIDImmun, advanced grant 249816), and the Imagine Foundation.
Authorship
Contribution: F.E.S. designed, conducted, and analyzed experiments with the assistance of A.G. and M.K.; S.M. designed, conducted, and analyzed experiments; C.A.J.V. and J.P.D.S. generated and provided Ncr1greenCre mice, provided tools, and provided valuable discussion; G.M. and A.F. interpreted and discussed data; F.E.S. and S.M. prepared the figures for the manuscript; F.E.S., S.M., and G.d.S.B. wrote the manuscript; F.E.S., S.M., A.F., J.P.D.S., and G.d.S.B. edited the manuscript; F.E.S. and G.d.S.B. designed and supervised the overall research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fernando E. Sepulveda, INSERM U1163, Imagine Institute, Hôpital Necker-Enfants Malades, F-75015 Paris, France; e-mail: fernando.sepulveda@inserm.fr; and Geneviève de Saint Basile, INSERM U1163, Imagine Institute, Hôpital Necker-Enfants Malades, F-75015 Paris, France; e-mail: genevieve.de-saint-basile@inserm.fr.

![Figure 1. Presence of NK-cell cytotoxic activity reduces HLH-like manifestations in mice. Rag2−/− Il2rg−/− and Rag2−/− mice were reconstituted with BM from Prf1−/− and control B6 donors. After 8 weeks, TPrf1−NKPrf1− (open bars/open squares), TPrf1−NKPrf1+ (gray bars/gray circles), and TPrf1+NKPrf1+ mice (black bars/black squares) were infected with 200 PFU of LCMV-WE. Clinical and biochemical parameters were determined after infection. (A) Survival, body weight, and body temperature. (B) White blood cell counts were analyzed on day 14 postinfection. (C) Red blood cell, hematocrit, and platelets counts on day 14 postinfection. (D) The time course of neutrophils counts. Data (mean ± standard error of the mean [SEM]) are representative of 3 to 4 independent experiments with at least 3 mice in each group. For survival experiments TPrf1−NKPrf1− (n = 10), TPrf1−NKPrf1+ (n = 9), and TPrf1+NKPrf1+ (n = 12) mice. Dotted lines represent minimal normal values for control mice. Statistical analysis was performed using log-rank test or 1-way ANOVA. **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/9/10.1182_blood-2014-09-602946/4/m_1427f1.jpeg?Expires=1769096726&Signature=d6vw~oO599J0kR9UW5nVWU4b9f24d1dQuJJlbJfk6w0jkc7ITZLSbEGt0N0plkkdMd2WfRZMA29mhc1zJsFXNOA2zB6AtbHNXjhGismmvOZVRuOhSR2RSn7Fu4kMg4TPoKPRpnt~L~9Ge4cXzoTqfM44zE9ZLZOMlcaggxgNjSgXXrhG7w0BB4peu2epz7p-ZBjTxzlgdOy4Sozj4sWOF32DqRJw2sSyMiNZ0tgcjozssU4PfGt-UMV1lcKu~S3MNMqTmhr7rRp1Mqa73RJjU~wu6Aj6AIlC99YW00KRXEPvmVEg2n9PS4NLtfBVCRe4D2AByOEvi0bCcUKmXSERbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal