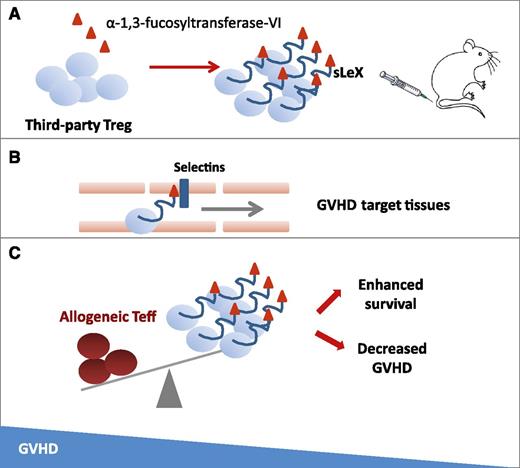
In this issue of Blood, Parmar et al report on preclinical data suggesting that use of ex vivo fucosylated third-party human regulatory T cells (Tregs) could be an effective strategy for prevention of graft-versus-host disease (GVHD).1
Fucosylation by α-1,3-fucosyltransferase-VI enzyme on ex vivo–expanded Tregs forming the sialyl Lewis X moiety (sLeX) on P-selectin glycoprotein ligand-1 (A) is a strategy to improve in vivo trafficking and persistence in GVHD target tissues (B), leading to a balance in favor of Tregs instead of allogeneic T effector (Teff) cells and to improved survival in treated mice (C).
Fucosylation by α-1,3-fucosyltransferase-VI enzyme on ex vivo–expanded Tregs forming the sialyl Lewis X moiety (sLeX) on P-selectin glycoprotein ligand-1 (A) is a strategy to improve in vivo trafficking and persistence in GVHD target tissues (B), leading to a balance in favor of Tregs instead of allogeneic T effector (Teff) cells and to improved survival in treated mice (C).
Over the past decade, the discovery of regulatory T cells (Tregs) has dramatically changed our understanding of autoimmune diseases.2 A genetically determined absence of Tregs leads to severe multiorgan autoimmune diseases (eg, the human immunodysregulation polyendocrinopathy enteropathy X-linked syndrome and the murine X-linked lymphoproliferative disease [scurfy mice]). Experimental ablation of Tregs at any time in life leads to “catastrophic autoimmunity” and death in 2 to 3 weeks. Thus, the concept of a Treg/T-effector cell balance in health and autoimmune diseases is of the utmost importance.
Effective prevention and treatment of graft-versus-host disease (GVHD) after allogeneic stem cell transplantation (allo-SCT) have always been very challenging and could be considered the Holy Grail in the field. The role of Tregs in GVHD pathophysiology was originally established in mice.3 The first clinical results of the treatment of patients with GVHD with human ex vivo–expanded Tregs was reported by Trzonkowski et al4 and suggested that the prophylaxis, rather than the treatment, of the acute GVHD late phase is more beneficial.3 In another study, Tregs prevented GVHD and promoted immune reconstitution after human leukocyte antigen–haploidentical allo-SCT.5 In the latter report, 2 of 26 evaluable patients developed acute GVHD of grade ≥2, whereas the majority remained free of clinically relevant GVHD. Interestingly, low-dose interleukin-2 was used for Treg enhancement in chronic GVHD.6,7 Finally, infusion of ex vivo–expanded Tregs in adults transplanted with umbilical cord blood proved to be safe8 ; however, this strategy proved to be insufficient because of the large donor-to-donor variability in Treg populations, which was responsible for their inability to reach a clinically meaningful dose in 20% of patients.
Based on this background, Parmar et al1 attempted to develop a novel approach to increase Treg potency. The authors hypothesized that ex vivo fucosylation could improve Treg homing and enhance their anti-GVHD efficacy. Thus, they added fucose to human Tregs, forming the sialyl Lewis X moiety on P-selectin glycoprotein ligand-1 to improve their trafficking pattern. The selectin pathway recruiter, α-1,3-fucosyltransferase-VI enzyme, significantly increased Treg surface fucosylation. In a xenogeneic GVHD mouse model, fucosylated Tregs showed prolonged periods of in vivo persistence, ameliorated GVHD, and improved survival (see figure).
There are several limitations to this approach, such as the schedule of Treg administration, the efficacy in GVHD target organs other than skin, the potential impact on established GVHD, the purity and complete characterization of the infused cell subsets, and the exact mechanism of action of Tregs (eg, long-term persistence vs differential migration into GVHD target organs). Despite these limitations, Parmar and colleagues’ results are appealing and can pave the way for further research in this direction. The first report on cancer and fucosylation (see figure) was published in 1979.9 More recently, it was shown that fucose-deficient hematopoietic stem cells have decreased self-renewal and aberrant marrow niche occupancy.10 In contrast, ex vivo fucosylation improved human cord blood homing and engraftment, because fucosylation of selectin ligands is critical for the rolling of primitive hematopoietic progenitor cells (see figure). The new study by Parmar et al1 now provides evidence that ex vivo fucosylation of other cell subsets such as Tregs can be an attractive method by which to improve GVHD prophylaxis. They also offer the rationale and impetus to test the effect of ex vivo fucosylation in other settings (tailored antiviral or antitumor cytotoxic T-lymphocyte generation, donor leukocyte infusion manipulation, and so forth), with the goal of improving the safety and efficacy of allo-SCT. The optimal dosing, schedule timing, cell numbers, mechanisms of action, and interactions with other regulatory cells (eg, natural killer T cells and mesenchymal cells) are yet to be established. A refined understanding of molecular events/cascades associated with fucosylation could eventually allow selective targeting of the GVHD and immune-mediated graft-versus-leukemia pathways. The study by Parmar et al1 is a step further in the right direction.
Conflict-of-interest disclosure: The authors declare no competing financial interests.