In this issue of Blood, Huang et al show that aberrant expression of TOX plays a central role in malignant survival, proliferation, and tumor formation in cutaneous T-cell lymphoma (CTCL).1
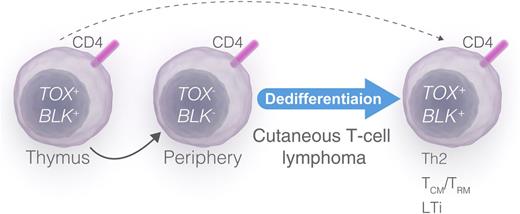
A proposed model of malignant dedifferentiation and TOX expression in CTCL.TOX and BLK become upregulated during thymic development of CD4+ T cells (left). In contrast, mature peripheral CD4+ T cells do not upregulate TOX on TCR stimulation and do not express BLK (middle). TOX- and BLK-positive malignant T-cell clones arise from a derailed thymic stem cell (dashed arrow) or as a result of TOX-dependent and/or TOX-independent dedifferentiation of malignant T cells or a precursor T cell (solid arrow). Dedifferentiation of the malignant T cell (or a precursor T cell) drives deregulated expression of transcription factors (including TOX), Scr kinases (including BLK), cytokines, and lymphangiogenic factor (right), leading to progressive disease and a mixed phenotype mimicking Th2, Th17, Th22, central memory/resident memory T cells (TCM/TRM), and LTi cells (right). Professional illustration by Luk Cox, Somersault 18:24.
A proposed model of malignant dedifferentiation and TOX expression in CTCL.TOX and BLK become upregulated during thymic development of CD4+ T cells (left). In contrast, mature peripheral CD4+ T cells do not upregulate TOX on TCR stimulation and do not express BLK (middle). TOX- and BLK-positive malignant T-cell clones arise from a derailed thymic stem cell (dashed arrow) or as a result of TOX-dependent and/or TOX-independent dedifferentiation of malignant T cells or a precursor T cell (solid arrow). Dedifferentiation of the malignant T cell (or a precursor T cell) drives deregulated expression of transcription factors (including TOX), Scr kinases (including BLK), cytokines, and lymphangiogenic factor (right), leading to progressive disease and a mixed phenotype mimicking Th2, Th17, Th22, central memory/resident memory T cells (TCM/TRM), and LTi cells (right). Professional illustration by Luk Cox, Somersault 18:24.
CTCL is a relatively rare and mysterious T-cell malignancy of unknown etiology. The key feature is clonal expansion of malignant T cells, but the pathogenesis is far from understood. The big question is what drives transformation from an indolent skin condition with a good prognosis and a normal life expectancy to a highly aggressive cancer with a poor prognosis. Here, Huang et al1 show that aberrant expression of thymocyte selection-associated high-mobility group (HMG) box gene TOX is associated with increased disease-specific mortality in patients with Sézary syndrome, a leukemic variant of CTCL. Importantly, TOX drives mitogenesis and survival of malignant T cells in vitro and tumor formation in vivo. The effect on cell cycle progression is notably mediated through a TOX-dependent repression of the 2 cyclin-dependent kinase inhibitors CDKN1B and CDKN1C, providing the first evidence of a direct link between TOX and these cell cycle regulators.1
TOX is a nuclear factor which is expressed in the thymus in a stage-specific and regulated manner and believed to play an important role during development of double-positive thymocytes.2 T-cell receptor (TCR)-mediated signaling drives TOX expression during positive selection, and mice deficient in TOX reveal a requirement for this factor in the development of all CD4+ T lineage cells, including conventional CD4+ T cells, FoxP3+ regulatory T cells, and natural killer T cells.3 Yet, the upregulation of TOX is transient and associated with thymic development but is not seen in mature, naïve CD4+ T cells.2 Malignant T cells in Sézary syndrome patients resemble healthy mature CD4+ cells and display a phenotype similar to that of central memory CD4+ T cells.4 The discovery by Huang et al1 of an ectopic expression and function of a thymic transcription factor (TOX) in malignant T cells strongly indicates that these cells are distinctly different from their healthy counterparts and have similarities with immature thymocytes. An aberrant TOX expression is not a unique feature of Sézary syndrome T cells. Patients with the more common variant of CTCL, mycosis fungoides, also display ectopic expression of TOX by malignant T cells. Interestingly, they also display aberrant expression of other thymic genes such as the B lymphoid kinase (BLK) and cancer-testis genes, which are not expressed in mature peripheral and skin resident T cells.5-7 In concert with the present study by Huang et al,1 these findings could suggest that malignant T cells either arise from a transformed thymic stem cell or become deregulated, “reopening” a thymic expression program (see figure). The fact that malignant T cells usually display a fully completed TCR rearrangement, a functional TCR CD3 complex (at least in the initial stages of the disease), and a phenotype similar to that of a mature T cell argues against an early thymic stem cell origin. Therefore, I propose that the aberrant expression of thymic and stem cell genes is a result of a dedifferentiation, leading to re-expression of TOX, BLK, and other developmental genes in the malignant T cell or its malignant precursor in the thymus, bone marrow, or peripheral lymphoid system (see figure). Whether TOX expression is a consequence of dedifferentiation or takes an active part in the transition remains to be investigated. As TOX engages in a multitude of cellular processes such as transcriptional regulation, multiprotein complex formation, and modulation of chromatin structures, it is likely that TOX is directly involved in deregulation of a number of signaling events in malignant T cells. Although malignant T cells resemble mature T cells, they often display a confused cytokine profile, expressing various mixtures of lineage-specific cytokines such as T helper (Th) type 2 cells (interleukin [IL]-4, IL-5, IL-13), regulatory T cells (IL-10, transforming growth factor β), Th17 (IL-17, IL-22), and Th26,8-10 and studies are warranted to identify whether TOX drives deregulated expression of cytokines as well as growth factors and lymphangiogenic factors.
In addition to the role in the thymus, TOX is essential for development of lymphoid tissue inducer (LTi) cells and peripheral lymphoid tissue such as lymph nodes and Peyer patches.3 Interestingly, LTi and malignant T cells produce many of the same cytokines and factors, raising the possibility that TOX expression during dedifferentiation drives malignant T cells toward an LTi-like phenotype (see figure). Because malignant T cells induce lymphoid vessel–like and lymph node–like structures in a xenograft mouse model of CTCL (N.O., unpublished data, January 2015), it may be speculated that aberrant TOX expression, in addition to the role in tumor growth reported by Huang et al,1 also plays a role in lymphoid dissemination of malignant T cells. This could also explain some of the dramatic effect of aberrant TOX expression on disease-specific mortality observed by Huang et al.1
Several strategies have been explored to enhance the efficacy of current treatments and to find new treatment options to improve survival and quality of life for patients with Sézary syndrome and other forms of advanced CTCL. The discovery of a novel driver of tumor growth calls for intensive research to clarify further the mode of action and the potential of TOX as a target for therapeutic intervention.
Conflict-of-interest disclosure: The author declares no competing financial interests.