Key Points
Loss of DGKε in endothelial cells induces cell death, impairs angiogenic responses, and leads to an activated and prothrombotic phenotype.
DGKE silencing in resting endothelial cells does not affect complement activation at their surface.
Abstract
Atypical hemolytic uremic syndrome (aHUS) is classically described to result from a dysregulation of the complement alternative pathway, leading to glomerular endothelial cell (EC) damage and thrombosis. However, recent findings in families with aHUS of mutations in the DGKE gene, which is not an integral component of the complement cascade, led us to consider other pathophysiologic mechanisms for this disease. Here, we demonstrate that loss of DGKε expression/activity in EC induces an increase in ICAM-1 and tissue factor expression through the upregulation of p38-MAPK–mediated signals, thus highlighting a proinflammatory and prothrombotic phenotype of DGKε-deficient ECs. More interestingly, DGKE silencing also increases EC apoptosis and impairs EC migration and angiogenesis in vitro, suggesting that DGKE loss-of-function mutations impair EC repair and angiogenesis in vivo. Conversely, DGKE knockdown moderately decreases the expression of the complement inhibitory protein MCP on quiescent EC, but does not induce complement deposition on their surface in vitro. Collectively, our data strongly suggest that in DGKE-associated aHUS patients, thrombotic microangiopathy results from impaired EC proliferation and angiogenesis rather than complement-mediated EC lesions. Our study expands the current knowledge of aHUS mechanisms and has implications for the treatment of patients with isolated DGKE mutations.
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a severe form of thrombotic microangiopathy (TMA) that affects primarily the kidney. It is characterized by the occurrence of endothelial damage and fibrin/platelet thrombi in the kidney microvasculature, leading to its typical triad of hemolytic microangiopathic anemia, thrombocytopenia, and acute renal injury.1 aHUS has a poor prognosis, with a 2% to 10% mortality rate, and about two-thirds of patients progress toward end-stage renal disease, and there is a high risk of recurrence of the disease after kidney transplantation.2 Over the past decade, many studies have highlighted the central role of complement alternative pathway dysregulations in the development of aHUS.3-5 Several mutations in genes encoding complement regulatory proteins (factor H,6 MCP,7 factor I,8 thrombomodulin9 ) or components of the alternative C3 convertase (C3,10 factor B11 ), as well as the presence of circulating inhibitory anti–factor H antibodies,12 have been shown to predispose to the development of aHUS. It is currently assumed that complement alternative pathway activation triggered mainly by infection or pregnancy13 leads to endothelial cell (EC) damage and TMA. These observations led to the development of complement-targeted therapies for the treatment of aHUS,14 and eculizumab, a monoclonal antibody directed against human complement component C5, has led to the reversal of thrombocytopenia and hemolysis, a remarkable improvement in the renal outcome of aHUS patients, and the resolution of extra-renal manifestations of aHUS (reviewed in reference 15). Nevertheless, a recent study identified in patients with aHUS new mutations in a gene unrelated to the complement pathway, the DGKE gene, which encodes the ε isoform of diacylglycerol kinase (DGKε).16 Complement activation in patients with DGKE mutations is variable,16,17 suggesting that DGKE loss of function may be in itself the main trigger of TMA. Little data are available regarding the role of DGKε in EC, which adds to the complexity of the physiopathology of DGKE-related aHUS.
Diacylglycerol kinases (DGKs) are intracellular lipid kinases that phosphorylate diacylglycerol (DAG) to phosphatidic acid (PA), thus terminating DAG signaling.18 The DGKε isoform phosphorylates and inactivates preferentially arachidonic acid–containing DAG (AADAG), and is thus mainly involved in the phosphatidylinositol cycle.19 DGKε lacks extra-enzymatic regulatory domains found in other DGK isoforms,20 which suggests that it may be constitutively active in physiologic conditions. In the kidney, DGKε has been found to be expressed in several cell types, including podocytes21 and glomerular ECs.16 Because AADAGs are known to activate protein kinase C (PKC),22 which has the potential to stimulate a broad range of signaling pathways, in particular MAP kinase–mediated signals,23 it is plausible that loss of DGKε in ECs may result in overactivation of PKC and of its downstream signaling networks, leading in turn to activation of various endothelial biological responses.24 However, the exact role of DGKε in the regulation of intracellular signaling pathways downstream of AADAG is still little described, and its function in the regulation of EC responses is unknown.
Here, we demonstrate that loss of DGKε in EC results in permanent overactivation of p38- and p44/42-MAP kinases, leading to a proinflammatory and prothrombotic state of these cells. DGKE silencing in EC also leads to increased apoptosis and impaired migration/angiogenesis. In addition, DGKE knockdown slightly modulates the expression of the complement regulatory protein MCP without the occurrence of C3 deposition on resting ECs. Collectively, our data define a model whereby loss of DGKε expression/activity in EC triggers the development of aHUS through complement-unrelated mechanisms, mainly the impairment of EC proliferation and angiogenesis, and they suggest that complement-targeted therapies may not be appropriate for patients with isolated DGKE mutations.
Materials and methods
Antibodies and reagents
Full details of antibodies and reagents used can be found in supplemental Methods, available on the Blood Web site.
Cell culture and siRNA knockdown
Primary human umbilical vein ECs (HUVECs) and human dermal microvascular ECs (HMECs) from single donors were purchased from Clonetics (Lonza) and cultured in complete endothelial growth medium (EGM-2; Lonza) and complete microvascular endothelial growth medium (EGM-2-MV; Lonza), respectively. Cells were subcultured and used at passage 4 or 5, and the surface expression of the endothelial marker CD31 was assessed after each passage (data not shown). Of note, we were unable to perform experiments in commercially available primary human glomerular ECs because in 3 batches of cells we tested, we could not detect CD31 expression, and because these cells rapidly acquired a fibroblastlike phenotype. A pool of 4 independent small interfering RNAs (siRNAs) targeting different sequences of DGKE mRNA and designed to achieve strong on-target knockdown with minimal off-target effects (“SMARTpool”) was purchased from Thermo Scientific, as was a pool of 4 nontargeting siRNAs, which were used as a negative control. HUVECs and HMECs were transfected with siRNAs (10 or 20 nM) using lipofectamine RNAiMAX (Life Technologies, Invitrogen) according to manufacturer’s instructions.
RNA isolation and quantitative real-time polymerase chain reaction
Total RNA was isolated from HUVECs using Trizol (Life Technologies). cDNA was generated using M-MLV-RT (Life Technologies) according to the manufacturer’s instructions. Real-time polymerase chain reaction (PCR) was performed using specific TaqMan Gene Expression Assays (Applied Biosystems) on a 7900HT real-time PCR system (Applied Biosystems). The HPRT gene was used to normalize the results.
Enzyme-linked immunosorbent assay
The human coagulation factor III/tissue factor Quantikine enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems) and the human von Willebrand Factor (VWF) ELISA kit (Abcam) were used for ELISA assays in culture supernatants, according to the manufacturer’s instructions.
In vitro migration assays
HUVECs were transfected with DGKE or control siRNAs, and after 24 hours, cells were harvested with trypsin/ethylenediamine tetraacetic acid and seeded in 24-well plates (100 000 cells/well). After another 24 hours, a linear wound was created in confluent cell monolayers by scratching with a pipette tip. After an additional 6-hour incubation, cell migration into the wound was assessed by microscopy (Olympus IX71 inverted microscope). Images (×20 magnification) were taken with an Olympus DP72 camera and viewed with CellSens Viewer acquisition software (Olympus, Rungis, France), and migration was measured using ImageJ software as the percentage of area covered by migrating cells in the initial wound. Results are expressed as the ratio between DGKE siRNA–transfected cells and control siRNA–transfected cells, from 4 independent experiments.
Tube formation assay
Twenty-four–well plates were coated with 175 µL per well of Matrigel basement membrane matrix (BD Biosciences), which was then allowed to polymerize for 30 minutes at 37°C. HUVECs transfected with DGKE or control siRNAs were seeded on top of the Matrigel (75 000 cells/well), and after 6 hours of incubation at 37°C, the network of tubes formed was observed under an inverted microscope (Olympus IX71, ×10 magnification). Five fields of view per condition were randomly photographed (Olympus DP72 camera and CellSens Viewer acquisition software, Olympus). The number of junctions in the capillary network was quantified using ImageJ software. Results are expressed as the ratio of the number of junctions per field between DGKE siRNA– and control siRNA–transfected cells.
Protein arrays
Protein arrays were performed using the Human Phospho-Kinase Array Kit (Proteome Profiler Array; R&D Systems), according to the manufacturer’s instructions.
Platelet adhesion assays
Human platelets were isolated from the blood of healthy volunteers as previously described.25 Briefly, blood was drawn in Vacutainer tubes (BD Diagnostics) containing sodium citrate, acid citrate, and glucose (ACD) and centrifuged at 170g for 20 minutes to separate the platelet-rich plasma. Platelet-rich plasma was further centrifuged for 10 minutes at 1500g and platelets were resuspended at 100 × 106/mL in M199 containing 1 μM prostaglandin E1 to prevent their activation. Platelets were then stained with 2.5 μmol/L calcein acetoxymethyl ester (calcein-AM; Life Technologies) for 15 minutes at 37°C in the dark. After washing, platelets were resuspended in M199 and preactivated with 0.5 U/mL thrombin for 10 minutes at 37°C. Thrombin was then inactivated with 2 U/mL hirudin for 10 minutes at 37°C and platelets were coincubated with siRNA-transfected HUVECs for 1 hour at 37°C (125 × 106 platelets/mL). After 3 washes in phosphate-buffered saline, adherent platelets were visualized by fluorescent microscopy on an Olympus IX71 inverted microscope (×10 magnification) with an Olympus DP72 camera using CellSens Viewer acquisition software (Olympus), and quantified using ImageJ software.
Statistical analyses
Statistical analyses were performed using the Kruskal-Wallis test and P values <.05 were considered significant.
Full details of other experimental procedures can be found in the supplemental Methods.
Results
DGKE knockdown in ECs induces ICAM-1, E-selectin, and tissue factor expression
Although DGKε was shown to be expressed in ECs from different vascular beds, including kidney glomerular ECs,16 its function in EC biological responses is still elusive. Here, we analyzed the role of DGKε in the regulation of EC activation and prothrombotic responses using a knockdown approach with specific siRNAs. Transfection of HUVECs and HMECs with DGKE siRNAs at 10 nM or 20 nM led to a dose-dependent decrease in DGKE mRNA expression of ∼50% and 60%, respectively, as assessed by quantitative PCR analyses (Figure 1A), resulting in a marked decrease in DGKε protein expression, as documented by western blot analysis (Figure 1B). Using flow cytometry, we found that DGKE knockdown in HUVECs and in HMECs induced a significant increase in ICAM-1 (Figure 1C) and a minimal but significant increase in E-selectin expression (P < .01, Figure 1D), reflecting activation of these cells. To verify that these EC responses were actually caused by the specific inhibition of DGKε expression, and not to an off-target effect of one of the siRNAs from the Smartpool, we analyzed ICAM-1 expression after DGKE knockdown using 2 independent siRNAs from the pool. Transfection of HUVECs with 20 nM of these siRNAs was able to achieve a 60% reduction in DGKE mRNA expression (supplemental Figure 1A) and again induced a significant increase in ICAM-1 expression as assessed by flow cytometry (P < .01) (supplemental Figure 1B).
DGKε knockdown promotes an activated phenotype of ECs. (A) HUVECs and HMECs were transfected with control siRNAs or DGKE siRNAs, and DGKE expression was analyzed after 48 hours at the mRNA level to assess knockdown efficiency. The bar graph shows the mean percentage of inhibition of DGKE mRNA expression (± standard error of the mean [SEM]) from at least 3 independent experiments. **P < .01, ***P < .001. (B) DGKε protein expression was also assessed in cells transfected with control or DGKE siRNAs using western blot analysis. Representative results from 3 independent experiments are shown. (C) ICAM-1 and (D) E-selectin expression at the surface of HUVECs and HMECs was evaluated by flow cytometry 48 hours after DGKE knockdown vs control siRNA–transfected cells. HUVECs stimulated for 4 hours with 100 U/mL TNFα as a positive control for activation. Representative histograms are shown, and the bar graphs illustrate the mean fold change in median fluorescence intensity (MFI) (± SEM) from 4 independent experiments. *P < .05, **P < .01.
DGKε knockdown promotes an activated phenotype of ECs. (A) HUVECs and HMECs were transfected with control siRNAs or DGKE siRNAs, and DGKE expression was analyzed after 48 hours at the mRNA level to assess knockdown efficiency. The bar graph shows the mean percentage of inhibition of DGKE mRNA expression (± standard error of the mean [SEM]) from at least 3 independent experiments. **P < .01, ***P < .001. (B) DGKε protein expression was also assessed in cells transfected with control or DGKE siRNAs using western blot analysis. Representative results from 3 independent experiments are shown. (C) ICAM-1 and (D) E-selectin expression at the surface of HUVECs and HMECs was evaluated by flow cytometry 48 hours after DGKE knockdown vs control siRNA–transfected cells. HUVECs stimulated for 4 hours with 100 U/mL TNFα as a positive control for activation. Representative histograms are shown, and the bar graphs illustrate the mean fold change in median fluorescence intensity (MFI) (± SEM) from 4 independent experiments. *P < .05, **P < .01.
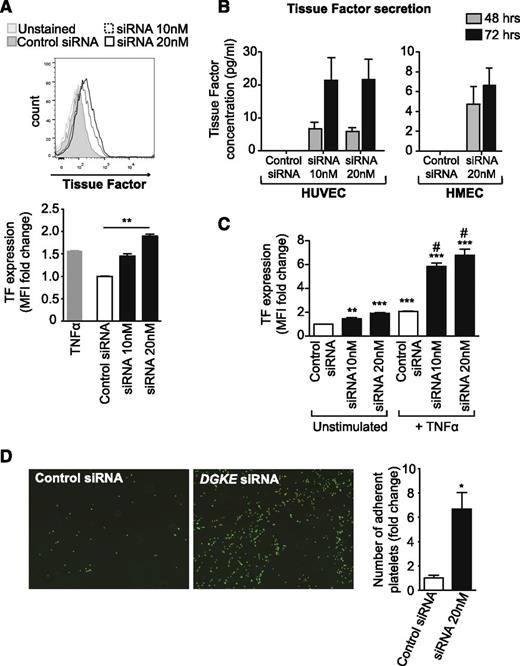
In addition to its effects on HUVEC and HMEC activation responses, DGKE knockdown also led to a marked increase in tissue factor (TF) expression at the surface of EC (P < .01) (Figure 2A), followed by the release of TF in cells supernatant 48 and 72 hours after transfection, suggesting a prothrombotic state of the cells (Figure 2B). DGKE knockdown also potentiated tumor necrosis factor-α (TNFα)-induced TF expression on HUVECs (P < .05) (Figure 2C), which suggests that a mutation or the absence of DGKε in ECs may amplify the procoagulant effects of inflammatory mediators. In contrast, knockdown of DGKE in ECs did not affect VWF cell-surface expression levels (supplemental Figure 2A), and even led to a decrease in soluble/secreted VWF in cells supernatants (supplemental Figure 2B). To determine whether loss of DGKε in ECs has prothrombotic effects, we analyzed platelet adhesion to ECs in vitro after DGKE knockdown. As is shown in Figure 2D, we found that DGKE siRNA transfection resulted in a significant increase in platelet adhesion compared with control siRNA–transfected cells. Collectively, these data suggest that DGKε mutations in ECs likely lead to the activation of these cells and the initiation of thrombosis—2 main features of aHUS.
DGKε deficiency leads to a prothrombotic phenotype of ECs. (A) HUVECs were transfected with 20 nM of control siRNA or with DGKE siRNA at 10 nM or 20 nM, and tissue factor (TF) cell-surface expression was analyzed 48 hours later by flow cytometry. HUVECs stimulated for 4 hours with 100 U/mL TNFα were used as a positive control. A representative histogram is shown, and the bar graph illustrates the mean fold change in MFI (± SEM) from 4 independent experiments. **P < .01. (B) The concentration of TF was measured by ELISA in culture supernatants from HUVECs and HMECs transfected with control or DGKE siRNAs 48 and 72 hours after transfection. The bar graphs represent the mean concentration of TF (± SEM) from 4 independent experiments. (C) HUVECs were transfected with control or DGKE siRNAs and cultured for 48 hours. TNFα was added for the last 4 hours of cell culture, and the expression of TF at the surface of cells was then analyzed by flow cytometry. The bar graph represents the mean fold change in MFI (± SEM) vs control siRNA–transfected and unstimulated cells, from 3 independent experiments. **P < .01 and ***P < .001 vs control siRNA–transfected unstimulated cells, and #P < .05 vs control siRNA–transfected and TNFα-stimulated cells. (D) Adhesion of freshly isolated calcein-AM–stained human platelets to HUVECs transfected with control or DGKE siRNAs (20 nM) was assessed by immunofluorescence microscopy. Representative photomicrographs show adhering platelets (green) on HUVECs (magnification ×40). The bar graph represents the mean fold change in the number of adherent platelets (± SEM) from 4 independent experiments.
DGKε deficiency leads to a prothrombotic phenotype of ECs. (A) HUVECs were transfected with 20 nM of control siRNA or with DGKE siRNA at 10 nM or 20 nM, and tissue factor (TF) cell-surface expression was analyzed 48 hours later by flow cytometry. HUVECs stimulated for 4 hours with 100 U/mL TNFα were used as a positive control. A representative histogram is shown, and the bar graph illustrates the mean fold change in MFI (± SEM) from 4 independent experiments. **P < .01. (B) The concentration of TF was measured by ELISA in culture supernatants from HUVECs and HMECs transfected with control or DGKE siRNAs 48 and 72 hours after transfection. The bar graphs represent the mean concentration of TF (± SEM) from 4 independent experiments. (C) HUVECs were transfected with control or DGKE siRNAs and cultured for 48 hours. TNFα was added for the last 4 hours of cell culture, and the expression of TF at the surface of cells was then analyzed by flow cytometry. The bar graph represents the mean fold change in MFI (± SEM) vs control siRNA–transfected and unstimulated cells, from 3 independent experiments. **P < .01 and ***P < .001 vs control siRNA–transfected unstimulated cells, and #P < .05 vs control siRNA–transfected and TNFα-stimulated cells. (D) Adhesion of freshly isolated calcein-AM–stained human platelets to HUVECs transfected with control or DGKE siRNAs (20 nM) was assessed by immunofluorescence microscopy. Representative photomicrographs show adhering platelets (green) on HUVECs (magnification ×40). The bar graph represents the mean fold change in the number of adherent platelets (± SEM) from 4 independent experiments.
DGKE knockdown induces EC apoptosis and impairs EC migration and angiogenic responses
EC damage is the main feature of aHUS, where it is classically induced as a result of complement activation. We thus analyzed whether DGKE mutations may directly induce EC damage and/or interfere with EC repair. We first evaluated EC angiogenic responses in a tube-formation assay in vitro. As is shown in Figure 3A, we found that DGKE knockdown induced a dramatic decrease in the angiogenic response of HUVECs in vitro (P < .01). We also evaluated EC migration using a wound-healing in vitro assay and, consistent with the impaired angiogenesis observed previously, we found that HUVEC migration was strongly decreased after DGKE knockdown (P < .05) (Figure 3B). Finally, we analyzed EC apoptosis by flow cytometry after staining with Annexin V and 7-AAD. As shown in Figure 3C, we found that DGKE knockdown in HUVECs and HMECs dramatically increased the number of apoptotic cells (Annexin V+ 7-AAD–) compared with control siRNA–transfected cells (P < .05). In contrast, EC proliferative responses (as assessed by Ki67 staining) were similar in DGKE siRNA–transfected EC vs controls (data not shown), suggesting that DGKε regulates angiogenesis through the modulation of EC survival and migration. Collectively, these results indicate that DGKε is critical for EC survival and repair, and that its mutation in aHUS patients will likely promote the development and maintenance of vascular damage.
DGKE knockdown in EC impairs their angiogenic responses in vitro by inhibiting migration and increasing apoptosis. (A) Control siRNA– or DGKE siRNA–transfected HUVECs (10 nM and 20 nM, respectively) were seeded on top of a Matrigel matrix and cultured for an additional 6 hours to allow the formation of tubelike structures. A representative photomicrograph of each condition is shown (×10). The bar graph shows quantitative analysis of the mean fold change in the number of junctions between tubes per field (± SEM) vs control siRNA–transfected cells from 4 independent experiments. (B) HUVECs were transfected with control or with 2 concentrations of DGKE siRNAs, and after 48 hours, a linear “scratch” was created in cell monolayers. Migration of cells into this wound was measured after 6 additional hours. Representative photomicrographs (×20) of wounds at 0 hours and after 6 hours are shown; the white lines highlight the linear wound for each group of cells. The bar graph shows the mean fold change in percentage of wound closure vs control siRNA–transfected cells (± SEM), from 3 independent experiments. (C) HUVECs and HMECs transfected with control or DGKE siRNAs were stained with Annexin V and 7-AAD 48 hours after transfection to evaluate apoptosis by flow cytometry. Representative dot plots are shown on the left, and the bar graph represents the mean percentage (± SEM) of apoptotic cells (Annexin V+ and 7-AAD−) from 4 independent experiments. *P < .05, **P < .01.
DGKE knockdown in EC impairs their angiogenic responses in vitro by inhibiting migration and increasing apoptosis. (A) Control siRNA– or DGKE siRNA–transfected HUVECs (10 nM and 20 nM, respectively) were seeded on top of a Matrigel matrix and cultured for an additional 6 hours to allow the formation of tubelike structures. A representative photomicrograph of each condition is shown (×10). The bar graph shows quantitative analysis of the mean fold change in the number of junctions between tubes per field (± SEM) vs control siRNA–transfected cells from 4 independent experiments. (B) HUVECs were transfected with control or with 2 concentrations of DGKE siRNAs, and after 48 hours, a linear “scratch” was created in cell monolayers. Migration of cells into this wound was measured after 6 additional hours. Representative photomicrographs (×20) of wounds at 0 hours and after 6 hours are shown; the white lines highlight the linear wound for each group of cells. The bar graph shows the mean fold change in percentage of wound closure vs control siRNA–transfected cells (± SEM), from 3 independent experiments. (C) HUVECs and HMECs transfected with control or DGKE siRNAs were stained with Annexin V and 7-AAD 48 hours after transfection to evaluate apoptosis by flow cytometry. Representative dot plots are shown on the left, and the bar graph represents the mean percentage (± SEM) of apoptotic cells (Annexin V+ and 7-AAD−) from 4 independent experiments. *P < .05, **P < .01.
DGKε regulates EC activation through p38-MAPK–mediated signals
DGK proteins are classically known to convert DAG to PA, thus downregulating DAG downstream signaling pathways, in particular PKC-mediated signals.18 To identify signaling pathways regulated by DGKε in ECs, we performed a phosphokinase array and thereby profiled the relative levels of phosphorylation of multiple kinases and some of their protein substrates in DGKE siRNAs–transfected HUVECs compared with control siRNA–transfected cells. As illustrated in Figure 4A, the array highlighted a most notable effect of DGKε in the regulation of MAP kinases phosphorylation/activation, including both p44/42-MAPK (ERK1/2) and p38-MAPK. Other phosphokinases were also markedly increased in DGKE siRNA–transfected cells, in particular MSK1/2 (a downstream target of MAP kinases26 ) and endothelial growth factor receptor. A few other phosphoproteins were downregulated, including Src family kinases (Fyn and Hck) and endothelial nitric oxide synthase (eNOS). The marked induction of MAPK activation upon DGKE knockdown was confirmed in additional experiments using western blot analyses (Figure 4B). Interestingly, although we found that DGKE knockdown in EC tended to increase PKC activity (Figure 4C), as expected, this effect seemed minimal compared with MAPK activation, suggesting that other intermediaries and/or amplification loops may be involved in DGKε-mediated regulation of MAPK signaling.
DGKE knockdown induces activation of p38- and p44/42-MAP kinases in ECs. (A) HUVECs were transfected with control or DGKE siRNAs (20 nM) and after 48 hours, a phosphokinase protein array was performed on cell lysates to analyze the relative expression of 46 individual kinases or some of their target proteins. The bar graph illustrates the fold change in pixel density for each protein of the array in DGKE siRNA–transfected cells vs control cells. (B) The expression of phospho-p44/42-MAPK (T202/Y204), total p44/42-MAPK, phospho-p38-MAPK (T180/Y182), and total p38-MAPK, as well as (C) phospho-PKC (S660), total PKC, and β-actin was examined in control or DGKE siRNA–transfected HUVECs using western blot analyses. Representative results of 3 independent experiments are shown.
DGKE knockdown induces activation of p38- and p44/42-MAP kinases in ECs. (A) HUVECs were transfected with control or DGKE siRNAs (20 nM) and after 48 hours, a phosphokinase protein array was performed on cell lysates to analyze the relative expression of 46 individual kinases or some of their target proteins. The bar graph illustrates the fold change in pixel density for each protein of the array in DGKE siRNA–transfected cells vs control cells. (B) The expression of phospho-p44/42-MAPK (T202/Y204), total p44/42-MAPK, phospho-p38-MAPK (T180/Y182), and total p38-MAPK, as well as (C) phospho-PKC (S660), total PKC, and β-actin was examined in control or DGKE siRNA–transfected HUVECs using western blot analyses. Representative results of 3 independent experiments are shown.
Because MAPK are well known to be critical mediators of EC responses associated with inflammation,27 we studied whether induction of p38-MAPK and p44/42-MAPK activation after DGKE knockdown was responsible for the induction of EC activation observed in the first part of this study. We transfected HUVECs with DGKE or control siRNAs, and after 6 hours, we added to the culture medium a specific pharmacologic inhibitor of p38-MAPK (SB203580, 10 μM) or of p44/42-MAPK (PD98059, 10 μM). After another 42 hours of culture, we analyzed the expression of ICAM-1 and E-selectin at the surface of cells by flow cytometry. As is shown in Figure 5A-B, we found that inhibition of p44/42-MAPK failed to suppress the induction of ICAM-1 and E-selectin expression induced by DGKE knockdown. In contrast, inhibition of p38-MAPK activity abrogated the induction of ICAM-1 and E-selectin on cells transfected with DGKE siRNAs. Collectively, these results demonstrate that loss of DGKε in ECs results in their activation through p38-MAPK-mediated mechanisms.
Loss of DGKε induces EC activation through p38-MAPK–mediated signals. HUVECs were transfected with 20 nM of control siRNA or DGKE siRNA, and a specific inhibitor of either p38-MAPK (SB203580, 10 μM) or p44/42-MAPK (PD98059, 10 μM), or dimethyl sulfoxide (DMSO) (vehicle) was added to the culture medium after 6 hours. (A) The expression of ICAM-1 and (B) E-selectin was analyzed 48 hours after transfection by flow cytometry. The bar graph represents the mean fold change in MFI (± SEM) from 3 independent experiments. #P < .05 vs control siRNA–transfected and DMSO-treated cells, *P < .05 vs DGKE siRNA–transfected and DMSO-treated cells.
Loss of DGKε induces EC activation through p38-MAPK–mediated signals. HUVECs were transfected with 20 nM of control siRNA or DGKE siRNA, and a specific inhibitor of either p38-MAPK (SB203580, 10 μM) or p44/42-MAPK (PD98059, 10 μM), or dimethyl sulfoxide (DMSO) (vehicle) was added to the culture medium after 6 hours. (A) The expression of ICAM-1 and (B) E-selectin was analyzed 48 hours after transfection by flow cytometry. The bar graph represents the mean fold change in MFI (± SEM) from 3 independent experiments. #P < .05 vs control siRNA–transfected and DMSO-treated cells, *P < .05 vs DGKE siRNA–transfected and DMSO-treated cells.
DGKε modulates the expression of the complement regulatory proteins MCP and DAF without inducing C3 deposition at the cell surface
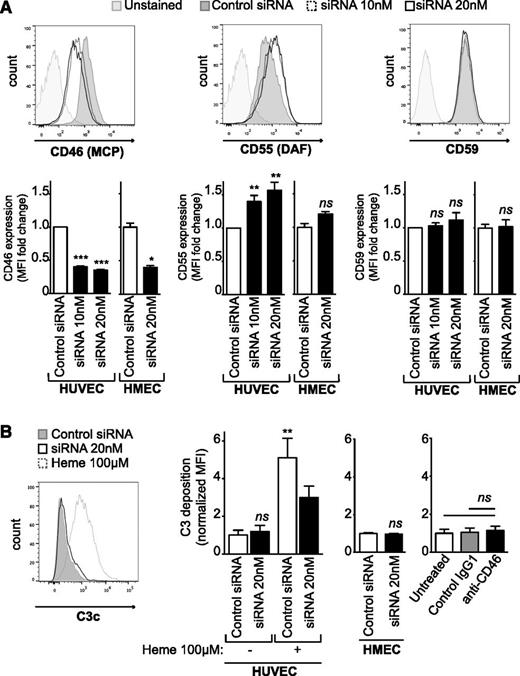
Because the complement alternative pathway is a major player in most forms of aHUS,14 we tested whether DGKε regulates the expression of complement inhibitory proteins on EC and/or binding of complement fragments to these cells. We thus analyzed by flow cytometry the expression of MCP (CD46), DAF (CD55), and CD59 at the surface of HUVECs and HMECs 48 hours after DGKE knockdown and found a marked decrease in MCP expression (P < .001) (Figure 6A, left panel). In contrast, DAF expression was slightly increased after DGKE silencing in HUVECs (P < .01), but this increase was not significant in HMECs (Figure 6A, middle panel). In addition, DGKE knockdown had no effect on CD59 expression on HUVECs and HMECs (Figure 6A, right panel). Because MCP is known to be a major inhibitor of complement activation at cell surfaces, mutated in as much as 15% of aHUS patients,1,7 we studied whether DGKε regulates C3b binding to ECs. HUVECs and HMECs were transfected with DGKE siRNAs, and after 48 hours, cells were incubated with fresh media containing 1:4 normal human serum (NHS) for 30 minutes, and C3b deposition at their surface was further evaluated by flow cytometry. ECs treated for 20 minutes with 100 μM of heme before incubation with NHS were used as a positive control for C3 deposition, as previously described.28 As is shown in Figure 6B, DGKE knockdown did not increase C3b binding to ECs. Similarly, blocking MCP activity at the surface of quiescent ECs using a specific inhibitory antibody did not alter C3b deposition, in contrast to observations on preactivated ECs (data not shown), or on ECs exposed to factor H–depleted serum or serum from aHUS patients with a C3 mutation, where C3 deposition has been shown to be enhanced.29 In addition, C3b deposition on ECs exposed to heme tended to decrease after DGKE knockdown compared with heme-exposed control siRNA–transfected cells. This apparent decrease may be caused by important cell injury as a result of the combination of DGKE knockdown and heme exposure. Collectively, these results suggest that DGKE deficiency is responsible for EC activation and damage but does not directly upregulate complement activation at the surface of these cells, at least in vitro.
DGKε regulates the expression of complement regulatory proteins but does not promote C3 deposition on ECs. (A) HUVECs and HMECs were transfected with 20 nM of control siRNA (shaded histogram) or with 10 nM (dashed histogram) or 20 nM (open histogram) of DGKE siRNA, and expression of MCP (CD46), DAF (CD55), and CD59 was analyzed 48 hours later by flow cytometry. Representative histograms are shown, and the bar graphs represent the mean fold change in MFI (± SEM) vs control siRNA–transfected cells from at least 4 independent experiments. **P < .01, ***P < .001. ns, not significant. (B) HUVECs and HMECs were transfected with control or DGKE siRNAs. After 48 hours, cells were incubated with 1:4 normal human serum (NHS) for 30 minutes at 37°C, and the deposition of C3c at their surface was evaluated by flow cytometry. Cells incubated for 20 minutes with 100 μM heme before NHS exposure were used as a positive control for C3 deposition, as described elsewhere.28 HUVECs treated for 30 minutes with a blocking anti-MCP antibody or a control IgG1 isotype (50 μg/mL) before NHS exposure were used to determine whether MCP blockade affects C3 deposition at the surface of ECs. A representative histogram is shown, and the bar graphs represent the mean fold change in MFI (± SEM) from at least 3 independent experiments. **P < .01.
DGKε regulates the expression of complement regulatory proteins but does not promote C3 deposition on ECs. (A) HUVECs and HMECs were transfected with 20 nM of control siRNA (shaded histogram) or with 10 nM (dashed histogram) or 20 nM (open histogram) of DGKE siRNA, and expression of MCP (CD46), DAF (CD55), and CD59 was analyzed 48 hours later by flow cytometry. Representative histograms are shown, and the bar graphs represent the mean fold change in MFI (± SEM) vs control siRNA–transfected cells from at least 4 independent experiments. **P < .01, ***P < .001. ns, not significant. (B) HUVECs and HMECs were transfected with control or DGKE siRNAs. After 48 hours, cells were incubated with 1:4 normal human serum (NHS) for 30 minutes at 37°C, and the deposition of C3c at their surface was evaluated by flow cytometry. Cells incubated for 20 minutes with 100 μM heme before NHS exposure were used as a positive control for C3 deposition, as described elsewhere.28 HUVECs treated for 30 minutes with a blocking anti-MCP antibody or a control IgG1 isotype (50 μg/mL) before NHS exposure were used to determine whether MCP blockade affects C3 deposition at the surface of ECs. A representative histogram is shown, and the bar graphs represent the mean fold change in MFI (± SEM) from at least 3 independent experiments. **P < .01.
Discussion
Over the past decade, great advances have been made in the understanding of aHUS mechanisms, the major breakthrough being the identification of a dysregulation of the alternative complement pathway as the central pathophysiologic event in this disease.14 This finding paved the way for the introduction of disease-specific complement-targeted therapies. However, 20% to 30% of aHUS patients do not exhibit any complement gene mutation, suggesting that the etiology of aHUS is diverse and that our understanding of its pathophysiologic mechanisms still remains incomplete. This was recently highlighted by the identification of recessive forms of aHUS linked to DGKE deficiency,16,17 which is not directly related to the complement cascade. Here, we studied the role of DGKε in EC responses and demonstrated that its expression is critical to maintain EC integrity and normal angiogenesis, because DGKε deficiency impairs EC angiogenic responses and promotes an activated and prothrombotic state of these cells. Our results suggest that DGKE deficiency will likely induce intrarenal TMA as a result of altered EC proliferation and angiogenesis rather than complement-induced damage to ECs.
Our findings show that loss of DGKε in ECs results in an overactivation of p38- and p44/42-MAPK pathways, probably in part through the relief of the DGKε inhibitory effect on PKC-mediated signals.23 However, we found only a moderate increase in PKC activation upon DGKE knockdown in ECs, which suggests that other mechanisms may be involved in DGKε-mediated regulation of MAPK activity and/or that amplification loops may enhance MAPK activation in this setting. We also find that overactivation of p38-MAPK signaling is responsible for increased ICAM-1 and E-selectin expression after DGKE knockdown. It is established that p44/42-MAPK is a central mediator of EC proliferation and survival,30 and it may therefore seem discrepant that DGKE knockdown leads to increased cell apoptosis and impaired angiogenic responses. Nevertheless, studies have shown that p38-MAPK can mediate EC apoptosis upon TNFα exposure, for example, and negatively regulates EC proliferation and angiogenesis.31 In addition, we hypothesize that DGKE silencing in ECs may induce the production of other factors that will in turn result in increased cell death and impaired migration. For instance, one interesting candidate is eNOS, which we found decreased in the protein array after DGKE knockdown. eNOS is well known to be critical for EC protection, and its inhibition has been suggested to induce EC dysfunction in various models.32 It is therefore likely that a decrease in its expression levels upon DGKE knockdown participates in damage to ECs.
Surprisingly, despite its important effects on EC activation, DGKE knockdown did not promote VWF expression in ECs. We even found soluble VWF expression to be reduced in the supernatant of DGKE siRNA–transfected ECs compared with control siRNA–transfected ECs, which may be a result of the important cell death induced by DGKE silencing. VWF secretion from Weibel-Palade bodies as a result of endothelial damage is a rapid event that is essentially mediated through intracellular calcium mobilization and cAMP33,34 and does not involve p38-MAPK–mediated signals, which explains why DGKE siRNA–transfected cells, although activated, do not overexpress VWF. In addition, although studies have shown that PKC mediates vascular endothelial growth factor–induced VWF release from ECs, additional unidentified intermediates are required for this process,35 and PKC overactivation by itself is not sufficient to promote VWF release,36 which explains why DGKε-mediated PKC regulation does not induce VWF secretion in our model. Nevertheless, although VWF expression was unchanged, we found platelet adhesion to be enhanced when HUVECs were transfected with DGKE siRNAs. Because VWF and P-selectin expression were unchanged at the surface of ECs after DGKE silencing (data not shown), we hypothesize that the increase in TF expression induced by DGKE knockdown may be directly responsible for adhesion of platelets, as observed in other models.25
In a first report, one patient with a recessive form of DGKE-associated aHUS presented a relapse of his disease while receiving eculizumab therapy, which suggests that DGKε deficiency induces EC damage without affecting the complement cascade itself.16 However, among the 19 cases of DGKE-associated aHUS reported thus far, 4 patients were found to have moderately decreased C3 levels.17,37 Three of these patients had isolated DGKE mutations, and one was found to have a concurrent mutation in the C3 gene.37 Our data demonstrate that EC damage induced upon DGKE silencing is likely responsible for the development of aHUS, independently of any effect of DGKε on the complement pathway. DGKE knockdown did not modify C3 deposition on resting ECs in vitro, despite a decrease in MCP expression at the surface of these cells. This could be explained by the fact that DGKE silencing in ECs also induces an increase in the expression of the other complement inhibitory protein DAF, which may counterbalance the effects of MCP loss. Moreover, blockade of MCP on resting ECs using a blocking antibody does not promote C3 deposition in vitro, suggesting that additional triggering events may be needed in MCP-associated aHUS patients. Conversely, DGKε-deficient apoptotic ECs may release microparticles that will promote C3 cleavage in the circulation and lead to the serum C3 consumption that has been described in some patients,17,38 and it could therefore be considered that transient complement activation may amplify in vivo EC damage as a result of DGKε inactivation. Finally, some of these patients with DGKE-associated aHUS may also carry mutations in other genes related to the complement system, as reported recently by Sanchez Chinchilla et al,37 which may influence the disease severity and explain the low C3 levels observed in some patients.
Patients with DGKE mutation exhibit different phenotypes ranging from aHUS to membranoproliferative glomerulonephritis with heavy proteinuria and nephrotic syndrome. DGKε has also been found to be expressed in podocytes and platelets,16 2 cell types that are involved in the development of glomerular microangiopathy. It is most likely that DGKε deficiency in ECs and podocytes disrupts the glomerular filtration barrier and would therefore explain the susceptibility of these patients to develop basement membrane abnormalities and heavy proteinuria.16,21 However, it remains unknown why patients with a DGKE mutation exhibit different phenotypes.
Altogether, our studies suggest that DGKε loss of function by itself leads to EC activation, apoptosis, and impaired angiogenesis, and ultimately to the development of aHUS, although complement deposition on EC does not seem to contribute to cell damage, at least in vitro, in this setting. DGKε deficiency should be added to the expanding list of mechanisms that lead to TMA, which already includes ADAMTS13 deficiency,39 complement dysregulation,14 VEGF deficiency,40 and Shiga-toxin–induced EC damage, among others.41
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr. Béatrice Charreau, INSERM Unité Mixte de Recherche S-1064, Nantes, France, for her expert technical assistance and for supplying some of the reagents used for this study.
This work was supported by INSERM Centre National de la Recherche Scientifique (ATIP-AVENIR) grant (F.F.); European Union's Research and Innovation FP7 grant 2012-305608 (EURenOmics) (V.F.-B.); and a Fondation Groupe des Assurances Mutuelles Agricoles (GROUPAMA) pour la Santé grant (M.N.).
Authorship
Contribution: F.F. and V.F.-B. initiated the research, conceived and designed the study, and edited the manuscript; S.B. and M.N. designed and performed the experiments, analyzed the data, and prepared the figures; S.B. wrote the manuscript; and L.T.R., M.F., and L.L. assisted with experiments.
Conflict-of-interest disclosure: F.F. and V.F.-B. are members of Alexion Pharmaceuticals’ National Advisory Board.
Correspondence: Fadi Fakhouri, INSERM UMR S-1064, Centre de Recherche en Transplantation et Immunologie, CHU de Nantes, 30 Boulevard Jean Monnet, 44093 Nantes cedex 01, France; e-mail: fadi.fakhouri@univ-nantes.fr.
References
Author notes
V.F.-B. and F.F. contributed equally to this work.
S.B. and M.N. are cofirst authors.

![Figure 1. DGKε knockdown promotes an activated phenotype of ECs. (A) HUVECs and HMECs were transfected with control siRNAs or DGKE siRNAs, and DGKE expression was analyzed after 48 hours at the mRNA level to assess knockdown efficiency. The bar graph shows the mean percentage of inhibition of DGKE mRNA expression (± standard error of the mean [SEM]) from at least 3 independent experiments. **P < .01, ***P < .001. (B) DGKε protein expression was also assessed in cells transfected with control or DGKE siRNAs using western blot analysis. Representative results from 3 independent experiments are shown. (C) ICAM-1 and (D) E-selectin expression at the surface of HUVECs and HMECs was evaluated by flow cytometry 48 hours after DGKE knockdown vs control siRNA–transfected cells. HUVECs stimulated for 4 hours with 100 U/mL TNFα as a positive control for activation. Representative histograms are shown, and the bar graphs illustrate the mean fold change in median fluorescence intensity (MFI) (± SEM) from 4 independent experiments. *P < .05, **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/6/10.1182_blood-2014-06-579953/4/m_1038f1.jpeg?Expires=1765936458&Signature=LY6eWDTjd2D57Le0ZEHPGhGAnCBj7nOJ4jNAc7MpXzTtNKlRKVv~RPTC4OaRyRCXJ9uJp06u-xN5SEV8drCKKokmti33BYZnNmHb7v8Q6QUH294AgAwGTg3irJ7dYG-qWTprViv6EQ~hj8zC7jWrDpdILVje52UH49sOo-POp5aZ~RxEZdN7ELX5eDZpHroIAbYMsBfpXuD2gu3RB04uaY0trG3vKmM-RSoa19kU-pJXWw5WFyH2P1xdTEsxBCXrQ88MP9HJMSAk~Q0SGHKxGSvMObe7JhWCx1rqcEHbRHYSyHyF31HErGy-5qNK8OiN7WMXYCCITFkSKzPM8H8zkw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal