In this issue of Blood, Nagai et al provide evidence that adult T-cell leukemia is hierarchically organized and sustained by a small population of transformed T memory stem cells.1
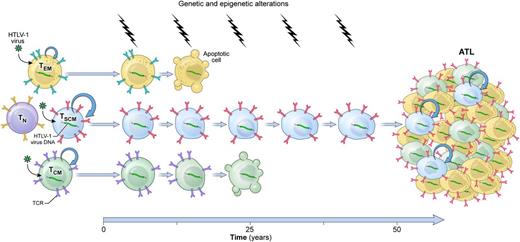
A model of HTLV-1–induced leukemogenesis. Longitudinal accumulation of genetic and epigenetic abnormalities in long-lived HTLV-1–infected TSCM cells leads to the development of ATL. TN, naïve T cells; TCR, T-cell receptor.
A model of HTLV-1–induced leukemogenesis. Longitudinal accumulation of genetic and epigenetic abnormalities in long-lived HTLV-1–infected TSCM cells leads to the development of ATL. TN, naïve T cells; TCR, T-cell receptor.
Adult T-cell leukemia (ATL) is a rare, aggressive CD4+ T-cell malignancy associated with human T-cell lymphotropic virus type 1 (HTLV-1).2 ATL occurs primarily in individuals from areas where HTLV-1 is endemic, such as southwestern Japan, the Caribbean islands, tropical Africa, South America, the Middle East, and northern Oceania.2 The precise mechanism of HTLV-1–induced leukemogenesis remains incompletely understood, but HTLV-1 infection appears to represent the first event of a multistep carcinogenic process.2 Indeed, only 3% to 5% of HTLV-1 carriers will develop the disease after several decades.2 Accumulation of genetic and epigenetic abnormalities in infected cells is thought to be necessary for ATL development.2 This classic multistep model of carcinogenesis likely involves a long-lived cell in which multiple genetic hits can cumulatively occur, but the identity of the cell type from which ATL originates is unclear.
According to the cancer stem cell (CSC) hypothesis, malignant tumors arise from cells with stem cell–like properties or directly from tissue-specific adult stem cells.3 A growing body of evidence indicates that, akin to other tissues, T cells are hierarchically structured with epitope-specific adult stem cells residing within the CD62L+ memory T-cell compartment.4 Among CD62L+ memory cells, T memory stem (TSCM) cells, a rare subset of cells displaying a naïve-like phenotype in conjunction with the expression of few memory markers such as CD95 and interleukin-2 receptor β, exhibit enhanced self-renewal and the multipotent capacity to reconstitute the full diversity of memory and effector T lymphocytes.5,6 Nagai et al provide compelling evidence implicating transformed TSCM cells as ATL initiating stem cells.
First, the authors show that HTLV-1 DNA integration cannot be measured in highly purified CD34+ lineage− hematopoietic stem and progenitor cells (HSPCs), indicating that contrary to certain types of mature lymphoid malignancies, ATL development is unlikely initiated in HSPCs. Next, they demonstrate that activation is a prerequisite for viral entry into T cells, as it induces the upregulation of several surface receptors involved in HTLV-1 binding and entry. Consistent with these findings, HTLV-1 provirus was virtually absent in naïve T cells but easily detectable in all memory subsets, including TSCM cells, from both HTLV-1 carriers and ATL patients. These findings underscore that, like its normal T-cell counterparts, ATL is composed of heterogeneous populations of cells that differ in their state of differentiation. The CSC model postulates that tumors behave as caricatures of their normal tissue counterparts.3 Nagai et al evaluate whether the functional hierarchy observed in a normal memory T-cell compartment was maintained in ATL. In this hierarchical organization, TSCM cells are the most undifferentiated subset, followed by CD62L+ central memory (TCM) cells and then by CD62L− effector memory (TEM) cells, which are prone to terminal differentiation.4,5 Using an in vitro culture assay in which highly purified subsets from ATL patients were grown in the presence of interleukin-7, the authors observed that the potential of a cell to differentiate into other subsets was progressively restricted, proceeding from TSCM to TCM and TEM cells. Indeed, TSCM cells were able to sustain themselves through a process of self-renewal while concomitantly giving rise to a progeny of TCM and TEM cells. On the other hand, neither TCM nor TEM cells generated TSCM cells. These findings indicate that ATL is organized in a manner that mirrors the functional architecture of the normal memory T-cell compartment. A hallmark of CSCs is their high tumorigenic potential compared with other neoplastic cells.3 Furthermore, tumors grown from CSCs are expected to contain the full phenotypic heterogeneity of the parental tumor.3 In a remarkable set of xenogeneic transplantation assays, Nagai et al demonstrate that among all T-cell subsets, only TSCM could efficiently reconstitute identical ATL clones in immunodeficient mice even when the theoretical numbers of transplanted HTLV-1–infected cells were as low as 390 cells. Strikingly, TCM cells did not show leukemogenic capacity even when inoculated at 10-fold higher numbers. Perhaps more importantly, transplanted ATL-TSCM cells recreated the full diversity of the original ATL. Serial transplantation experiments, a gold standard assay to evaluate cellular stemness,3 were not performed in this current study. Nevertheless, the data provided by Nagai et al highly support a model of leukemogenesis in which ATL is initiated and sustained by a minority of multipotent, self-renewing leukemic TSCM cells (see figure).
These findings provide, for the first time, a link between TSCM cells and a T-cell malignancy and underline how the remarkable longevity of TSCM cells can be hijacked for cell transformation. Recent data identifying CD4+ TSCM cells as a core element of the HIV reservoir offer another vivid example of how the stem cell–like qualities of this subset might be exploited to sustain a disease.7 The recognition of TSCM cells as central players in the maintenance of HTLV-1 and HIV diseases, however, raises the new exciting possibility of targeting this cell population for viral and ATL eradication. Targeting molecular pathways, such as Wnt/β-catenin signaling, that are essential for TSCM self-renewal and long-term survival8 might represent a new therapeutic strategy to disrupt these viral reservoirs.
Rather than the Achilles’ heel, the stem cell–like characteristics of TSCM cells are a valuable asset for immunotherapeutic interventions aiming at eliciting durable cellular immunity. For instance, the efficient induction of long-lasting TSCM cells by the yellow fever vaccine, one of the most successful antiviral vaccines ever developed, might be a key component for its particular efficacy.9 Long-term persistence of transferred T cells is also fundamental for the success of adoptive immunotherapies, as it correlates with objective antitumor responses in numerous clinical trials.4 TSCM cells have demonstrated an extraordinary antitumor efficacy in preclinical models of T-cell therapy, and tumor-redirected TSCM cells are actively pursued for clinical translation.5,8 Although the susceptibility of HTLV-1–infected TSCM cells to transformation warrants some consideration in using retrovirally engineered TSCM cells, high-resolution tracking of individual gene-modified TSCM clones over a decade did not show the emergence of clonal dominance.10 These findings indicate that TSCM cell gene engineering is relatively safe, adding some brightness to TSCM cells’ new dark side.
Conflict-of-interest disclosure: The author declares no competing financial interests.