Abstract
Warm antibody hemolytic anemia is the most common form of autoimmune hemolytic anemia. When therapy is needed, corticosteroids remain the cornerstone of initial treatment but are able to cure only a minority of patients (<20%). Splenectomy is usually proposed when a second-line therapy is needed. This classical approach is now challenged by the use of rituximab both as second-line and as first-line therapy. Second-line treatment with rituximab leads to response rates similar to splenectomy (∼70%), but rituximab-induced responses seem less sustained. However, additional courses of rituximab are most often followed by responses, at the price of reasonable toxicity. In some major European centers, rituximab is now the preferred second-line therapy of warm antibody hemolytic anemia in adults, although no prospective study convincingly supports this attitude. A recent randomized study strongly suggests that in first-line treatment, rituximab combined with steroids is superior to monotherapy with steroids. If this finding is confirmed, rituximab will emerge as a major component of the management of warm antibody hemolytic anemia not only after relapse but as soon as treatment is needed.
Introduction
Autoimmune hemolytic anemia (AIHA) is an uncommon acquired disorder in which autoantibodies directed against self-red blood cell membrane antigens lead to their accelerated destruction. The estimated incidence of AIHA in adults is 0.8-3 per 100 000 per year, with a mortality rate of 11%.1 The diagnosis is based on the presence of a hemolytic anemia with a positive direct antiglobulin test (or Coombs test) and on the absence of any other hereditary or acquired cause of hemolysis, although direct antiglobulin test-negative cases are not quite uncommon (5% of 308 cases of AIHA recently reported by the Gruppo Italiano Malattie EMatologiche dell'Adulto [GIMEMA]).2 Among AIHAs, AIHA caused by warm antibody (wAIHA) accounts for 70% to 80% of all cases in adults and for almost 90% of the cases in children.3 wAIHA can be either primary (or idiopathic) or associated in at least half of the cases with an underlying disease (lymphoproliferative disorder, 20%; autoimmune disease, 20%; infections; and tumors) and, in the latter case, is classified as secondary wAIHA.3
The management of wAIHA has long been and still remains mainly empirical or based on retrospective uncontrolled studies.1,4,5
When therapy is necessary, corticosteroids are usually given as first-line treatment. A response to corticosteroids is observed in 70% to 85% of the cases and generally occurs within the first 3 weeks of treatment.3 However, only one third of the patients remain in remission without therapy 1 year later and <20% are cured by steroids alone. The proportion of patients needing second-line therapy has been poorly assessed, with figures ranging from 20% to 30% up to 56%.1,2 Intravenous immunoglobulins, cyclosporin, mycophenolate mofetil, azathioprine, cyclophosphamide, and emergency treatment of the underlying disorder should all be considered in the rare life-threatening wAIHAs and/or in the absence of response to corticosteroids.6,7 Splenectomy has long been the main and preferred second-line option, with a sustained response rate of ∼ 60% to 70%, although secondary cases seem less responsive than idiopathic forms.8,9 The cure rate is estimated to be 20%. The role and best timing of splenectomy are currently controversial as alternatives such as rituximab are now available.
How does rituximab work?
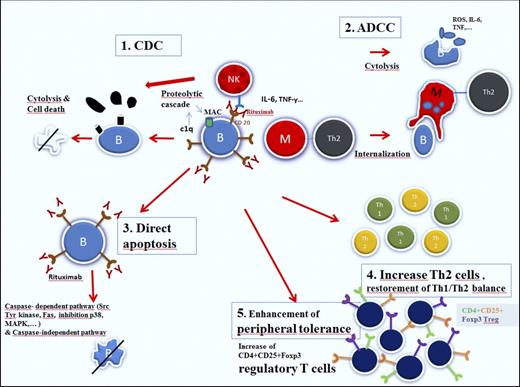
Rituximab is a chimeric human/murine monoclonal antibody directed against the CD20 antigen, expressed on the surface of precursor and mature B lymphocytes, but not on hematopoietic stem cells, very early B cells, and mature plasma cells (Figure 1). This selected target population explains the low toxicity profile of the molecule.10
Mechanisms of action of rituximab (reproduced from Dierickx et al24 with permission).
Mechanisms of action of rituximab (reproduced from Dierickx et al24 with permission).
In addition to their role in production and secretion of autoantibodies, B cells also contribute to the pathogenic process in immune-mediated disorders by producing inflammatory cytokines and by acting as antigen-presenting cells, leading to T-cell activation. Recent findings have also pointed to a major role of different T-cell subtypes in the onset and progression of different autoimmune disorders, especially CD4+CD25+FOXP3 regulatory T cells (Tregs). These Tregs are key players in the process of immunologic self-tolerance, with decreased levels being associated with autoreactivity and deterioration of autoimmune disorders, including AIHA.11 Another important T-cell player involved in the autoreactive process is the Th17 cell, a subset of CD4+ T helper cells. By secretion of interleukin-17, these cells also contribute to the autoimmune reaction.12 Finally, in patients with autoimmune disorders, there is a disturbed proinflammatory Th1/anti-inflammatory Th2 balance in favor of Th1 lymphocytes.13
By binding to CD20, rituximab has been shown to induce apoptosis of (both malignant and normal) CD20-positive B lymphocytes through different mechanisms, including antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, direct apoptotic activity, disturbed T-cell activation, and possible vaccinal effects.14 The latter effect, which has been demonstrated in follicular lymphoma patients, is based on the development of a specific cellular response.15 In patients treated for wAIHA, this effect has not been demonstrated, although the observation of delayed responses with rituximab may be partially explained by this effect. Initially it was hypothesized that B-cell depletion was responsible for the impressive response rates in a large proportion of patients presenting with different immune-mediated disorders. However, it soon became clear that B-cell depletion occurs in all patients treated with rituximab, whether they responded or not, requiring additional explanations.16 The effect of rituximab on the different T-cell subsets in immune-mediated hematologic disorders have been mainly reported in patients presenting with immune thrombocytopenia (ITP). In this disorder, characterized by the immune mediated destruction of platelets, rituximab has shown to be associated with a lasting and pronounced T-cell suppressive polarization, including elevation of Tregs and restoration of the Th1/Th2 balance, but only in responding patients.17-19 In addition, rituximab also induces downregulation of CD40 and CD80 on B cells, leading to further disturbed T-cell activation.20,21 Finally, it has been suggested that rituximab may lead to macrophage Fc-receptor blockade by rituximab-opsonized B cells and hence reduction of platelet destruction in the spleen, a mechanism referred to as the “immune complex decoy hypothesis.”22 Barcellini et al23 investigated the association between the clinical response to low-dose rituximab and cytokine production in patients with (both warm and cold types) AIHA. In this study, the authors found a decrease in interferon-γ, interleukin-12, and tumor necrosis factor-α (all TH1 cytokines) levels in patients responding to rituximab, consistent with restoration of the Th1/Th2 balance.
Can we anticipate which patients will be responsive to rituximab?
Several case reports and retrospective series have shown that treatment of patients with relapsed/refractory wAIHA with rituximab is associated with overall response rates of about 70% to 80%, with a median duration of response of ∼1 to 2 years (Table 1). However, similar to the use of steroids in this disorder, relapses are frequent.24 Median time to response is 4 to 6 weeks following the first rituximab dose, although responses within the first week (probably due to the immune complex decoy hypothesis) and after 3 months are not uncommon. A major drawback of these conclusions is the fact that comparison between different series is very difficult given the huge heterogeneity in criteria used to define responses.
Clinical evidence evaluating the efficacy of rituximab in the treatment of wAIHA
| First author (year) . | Study type . | wAIHA type . | Setting . | Number of patients . | Age (years)* . | Previous Splx . | ORR/CRR . | Response duration . |
|---|---|---|---|---|---|---|---|---|
| Zecca (2003) | P | M | R/R | 14 | 0-14 | 13 | 87/NR | 7-27+ |
| Narat (2005) | R | M | R/R | 11 | 18-81 | 5 | 64/27 | 2-20 |
| D’Arena (2007) | R | PW | R/R | 11 | 23-81 | 9 | 100/73 | 1-96+ |
| Bussone (2009) | R | M | R/R | 27 | 15-81 | 22 | 93/30 | NR |
| Dierickx (2009) | R | M | R/R | 36 | 1-87 | 19 | 79/47 | 1-year PFS = 72% |
| Peñalver (2010) | R | M | R/R | 27 | 20-86 | 13 | 77/61 | 6+(if CR) |
| Barcellini (2013) | P | PW | F;R/R | 18 | 19-79 | 0 | 90/60 | 36+ |
| Maung (2013) | R | M | R/R | 34 | 14-83 | 3 | 71/26 | 6-60 |
| Birgens (2013) | P | M | F | 32 | 35-90 | 0 | 75% CR at 12 mo | 36+ |
| Roumier (2014) | R | M | R/R | 25 | 30-76 | NR | 80/NR | 50% relapse after 14 ± 8 months |
| Barcellini (2014) | R | PW | R/R | 32 | 0-95 | NR | 81/53 | NR |
| First author (year) . | Study type . | wAIHA type . | Setting . | Number of patients . | Age (years)* . | Previous Splx . | ORR/CRR . | Response duration . |
|---|---|---|---|---|---|---|---|---|
| Zecca (2003) | P | M | R/R | 14 | 0-14 | 13 | 87/NR | 7-27+ |
| Narat (2005) | R | M | R/R | 11 | 18-81 | 5 | 64/27 | 2-20 |
| D’Arena (2007) | R | PW | R/R | 11 | 23-81 | 9 | 100/73 | 1-96+ |
| Bussone (2009) | R | M | R/R | 27 | 15-81 | 22 | 93/30 | NR |
| Dierickx (2009) | R | M | R/R | 36 | 1-87 | 19 | 79/47 | 1-year PFS = 72% |
| Peñalver (2010) | R | M | R/R | 27 | 20-86 | 13 | 77/61 | 6+(if CR) |
| Barcellini (2013) | P | PW | F;R/R | 18 | 19-79 | 0 | 90/60 | 36+ |
| Maung (2013) | R | M | R/R | 34 | 14-83 | 3 | 71/26 | 6-60 |
| Birgens (2013) | P | M | F | 32 | 35-90 | 0 | 75% CR at 12 mo | 36+ |
| Roumier (2014) | R | M | R/R | 25 | 30-76 | NR | 80/NR | 50% relapse after 14 ± 8 months |
| Barcellini (2014) | R | PW | R/R | 32 | 0-95 | NR | 81/53 | NR |
This table excludes series describing the use of rituximab in patients with only secondary wAIHA. CR, compete response; CRR, complete response rate; F, frontline; M, mixed primary and secondary wAIHA; NR, not reported; ORR, overall response rate; P, prospective; PW, primary wAIHA; PFS, progression-free survival; R, retrospective; R/R, relapsed or refractory; splx, splenectomy; wAIHA, warm type autoimmune hemolytic anemia.
Age of all included patients (warm + cold type).
In 5 studies that included ≥30 patients, factors predicting for a response to rituximab were analyzed.2,23,25-27 In 4 studies, responses in wAIHA and cold agglutinin disease (CAD) were compared and were found superior in wAIHA in 3 studies,2,23,25 whereas no difference in response rate was observed in the study of Peñalver et al. In the GIMEMA study, this difference was noted in patients given low-dose rituximab, whereas no significant difference in response rate was found after treatment with a conventional dose of rituximab.2 In addition, in the same study, an increased response rate was associated with young age and with a shorter interval between treatment and diagnosis only in patients given low-dose rituximab. In GIMEMA, patients were given a conventional dose of rituximab, and in the 4 other publications, a difference in outcome (generally in response rate) could not be identified with respect to age, sex, duration of the disease, previous splenectomy, presence of an underlying disorder, association of rituximab with other immunosuppressive drugs, or response to previous therapies.
Thus, at this time, no validated pretreatment clinical or biochemical parameter consistently predicts outcome following rituximab treatment, except for a better response rate in wAIHA compared with CAD and, possibly, younger age and early treatment in patients given low-dose rituximab.2,22 In a recently published meta-analysis of 21 studies younger age and wAIHA were confirmed as predictive factors for overall and complete response rate following rituximab therapy.28
Rituximab in some special presentations of wAIHA
Chronic lymphocytic leukemia-associated AIHA
Autoimmune cytopenias are well-known complications of chronic lymphocytic leukemia (CLL), AIHA being the most common with an estimated incidence of up to 10%.29 CLL patients with AIHA seem to have a better prognosis compared with those with advanced stage (Binet C) showing anemia due to bone marrow infiltration, but have a worse survival compared with early stage (Binet A) patients without anemia.30,31 For patients diagnosed with CLL-associated wAIHA, first-line therapy consists of steroids, similar to primary wAIHA. In case of failure, these patients may respond to rituximab-containing immunochemotherapy, with RCD (rituximab, cyclophosphamide, and dexamethasone) being the most successful regimen leading to overall response rates of 90% and a complete response rate up to 60%, with durable responses in a significant proportion of patients.32,33 Recently Quinquenel et al34 reported on 26 patients with CLL-associated AIHA treated with a combination of bendamustine and rituximab, leading to overall response rates of 81%, with a median duration of response of 28.3 months. Interestingly, half of the patients were previously treated with RCD, making comparison between both regimens possible. In the majority of cases, bendamustine and rituximab treatment was associated with a longer response duration compared with prior RCD treatment.
Evans syndrome
Evans syndrome (ES) is characterized by the sequential or simultaneous occurrence of AIHA and ITP. Treatment with rituximab is also efficacious in patients with ES, as shown by a French retrospective study: of 11 patients, 9 responded (5 complete and 4 partial),35 whereas in 8 patients, CLL-associated ES combination treatment with RCD induced 6 responses.33
Primary immunodeficiency-associated AIHA
Autoimmune cytopenias are a common feature in many primary immunodeficiencies, including common variable immunodeficiency. Treatment with rituximab in common variable immunodeficiency-associated autoimmune cytopenias was evaluated in a recent French retrospective multicenter study, where it was given to 33 (mainly adult) often heavily pretreated patients including 5 patients with AIHA and 7 with ES (no information on type of AIHA available). In this setting, rituximab appeared to be highly effective (4 of 5 complete responses in AIHA) and relatively safe, especially taking into account the vulnerability to infections in this population.36
AIHA in patients with systemic autoimmune disorders
In a recently published French single center study including 60 cases with wAIHA, 13% of all and 21% of secondary cases were associated with an underlying autoimmune disorder, mainly systemic lupus erythematosus (SLE).37 It has been estimated that AIHA occurs in up to 10% of SLE patients during their disease course, with most AIHA cases being diagnosed at the onset of the disease.38 Several trials with rituximab have been conducted in patients with SLE, showing significant improvement in hematologic complications, although 2 randomized trials failed to demonstrate a significant activity of rituximab on the underlying autoimmune disorder with or without renal involvement.39
Transplantation-associated AIHA
Allogeneic hematopoietic stem cell transplantation (HSCT) is associated with a high rate of complications, mainly including infections and graft-versus-host disease. The latter complication refers to an immunologic response, targeting cells and organs of the patient and explains the alloimmune cytopenias, including hemolytic anemia following allogeneic HSCT. On the other hand, lymphocyte depletion of the donor graft (to prevent graft-versus-host disease) can also lead to, probably due to removal of CD4+CD25+FOXP3 Tregs, autoreactive hemolytic anemia. In addition, de novo autoimmune cytopenias are also observed following autologous HSCT.40 The incidence of immune cytopenias following allogeneic HSCT, including cord blood transplantation, is estimated to be 2% to 6%. In a recently published large retrospective analysis from the London King’s College Hospital including 533 adult patients, overall incidence of allogeneic HSCT-associated (mainly warm type) AIHA was 3.6%. Response to first-line treatment with steroids was very poor (only 10%), requiring further immunosuppressive treatment in the majority of patients. Adding rituximab led to complete remission in 46% of the patients.41
Optimal dose of rituximab and duration of treatment
The optimal rituximab dose in AIHA and other autoimmune cytopenias has not been established. Initially, rituximab was used following the classical lymphoma protocol, consisting of 4 weekly doses of 375 mg/m2 per week.42 However, given the fact that the amount of B cells in a disturbed immune system is considerably lower compared with the high malignant B-cell burden in most lymphoproliferative B-cell disorders, lower doses of rituximab have been explored in the treatment of autoimmune cytopenias, especially in ITP. In a recently published prospective trial, Barcellini et al23 investigated the use of low-dose rituximab in 24 patients with warm- and cold-type AIHA. Rituximab was given in a fixed dose of 100 mg/week for 4 consecutive weeks. Overall response rate and relapse-free survival (RFS) at 12 months were both 100% in the warm type, with an estimated RFS at 2 years of 81%. An update of this study with longer follow-up confirmed the high sustained response rates with RFS in the whole group of 68% at 36 months.43 However, in the recently published GIMEMA study (that probably included the patients previously presented by Barcellini et al), the relapse rate of patients treated with low-dose rituximab (6 of 16) was significantly superior to the relapse rate observed after full-dose rituximab (2 of 42).2 In ITP, 2 trials have been conducted with low-dose rituximab, showing that the level and duration of B-cell depletion and the response rates seemed similar to cases treated with conventional dose of rituximab, although responses may be less durable.44,45
Response duration in patients with AIHA is very variable, with a median response of ∼1 year, although a very wide range is observed. Recent studies with longer follow-up show that responses up to 3 years are sustained in a proportion of patients (up to 30%).26,43 Whether some patients can be considered cured after rituximab administration is not clear, given the lack of long-term follow-up in most published series. Maintenance therapy with rituximab aiming to prolong duration of response has not been investigated in this setting. As a substantial fraction of patients benefits from a prolonged response after a first treatment with rituximab, maintenance assessment should better concentrate on relapsing patients unless the wAIHA occurs as a complication of an underlying condition known to benefit from maintenance with rituximab. Patients who, after successful treatment with rituximab, maintain a response at the price of prolonged low-dose steroids or other immunosuppressive treatments, or who poorly tolerate such a prolonged treatment, are also candidates for assessment of rituximab maintenance.
Should rituximab be used up front in patients with wAIHA?
Two recent prospective studies have confirmed the value of rituximab as first-line treatment. Barcellini et al43 treated 8 newly diagnosed patients with a fixed dose of rituximab of 100 mg for 4 weekly infusions. However, interpretation of responses is difficult given the low number of patients and the mixture of cases with wAIHA and CAD. More information is obtained from the first prospective randomized phase 3 trial conducted in Denmark, including 64 patients with newly diagnosed wAIHA.46 Following inclusion, patients were randomized to receive prednisolone with or without rituximab 375 mg/m2 per week for 4 consecutive weeks. Combination therapy was associated with an increased response rate (75% vs 36% at 12 months) and a longer relapse-free survival (70% vs 45% at 36 months) compared with monotherapy with steroids, without additional adverse events. As any clinical study, the Danish work has its limitations: the number of patients included in this study (32 in each arm) is relatively low, even if few clinical studies could accrue 64 patients with wAIHA and even if this is compensated to a large extent by the magnitude of the benefit in favor of patients given rituximab. The poor accessibility to rituximab as first-line therapy in this indication may limit its widespread use in many countries. In addition, some objectives of the study could not be met. In particular, there was no reduction in blood transfusion need or in the number of splenectomies in favor of patients assigned to rituximab. This provocative study should encourage additional clinical trials aimed at confirming the data, at identifying the patients who benefit from an early use of monoclonal antibodies, and at assessing if upfront rituximab could have a steroid-sparing effect, as similar doses of steroids were given in both arms of the Danish study. This point is particularly relevant as adult patients with wAIHA are relatively old (39% were ≥65 years of age in the GIMEMA study), and as comorbidities are commonly associated with wAIHA. A longer follow-up of the patients accrued in the Danish study showing that the benefit in favor of rituximab is maintained over time could also contribute to modify our first-line treatment policy in wAIHA. Meanwhile, upfront use of rituximab could be considered in patients who prove intolerant to steroids. The Danish data do not support the use of rituximab as an emergency treatment of severe cases of wAIHA, as the response rate of patients given upfront rituximab is not increased with respect to controls given steroids alone during the first 3 months of treatment.
Splenectomy or rituximab?
Although wAIHA is a chronic disorder, not all patients will need a second-line treatment. In a large French hospital a second-line therapy was given in 56% of 60 patients and in the GIMEMA study in 40% of 174 patients.2,37 In both studies, the proportion of patients offered a splenectomy was relatively low: in the French study, only 9 patients (15%) underwent splenectomy, whereas 28 (46.5%) patients were given rituximab at some stage of the disease. In the GIMEMA study, 26 patients with wAIHA (15%) underwent splenectomy, whereas 32 (18%) received rituximab. Whether the relatively low proportion of patients offered a splenectomy in both studies results from the use of rituximab is unknown because the proportion of patients with wAIHA undergoing splenectomy before the advent of rituximab has been poorly assessed. Of note, only 2 patients in the rituximab era underwent splenectomy after failure of first-line steroids (vs 19 who were treated with rituximab), whereas 7 were offered splenectomy at a later stage of the disease, which suggests that therapy with rituximab prior to (or instead of) splenectomy tends to become the standard of care, at least in that large French hospital.
Response rates to splenectomy and to rituximab seem equivalent even if no prospective study is available comparing the success rates of both approaches. In the French study, 9 patients underwent splenectomy: 6 achieved complete response (CR) and 3 achieved a partial response (PR). In the same study, 20 of 25 patients given rituximab achieved a PR or CR. Similar response rates were found in the GIMEMA study: of 26 patients offered a splenectomy, 20 (77%) responded (19 CRs and 1 PR) compared with 26 of 32 (81%) patients treated with rituximab, including 17 CRs and 9 PRs.
Duration of response between both therapeutic options does not seem very different. Splenectomy is associated with a 67% long-term response and a cure rate of ∼20%.1 These numbers seem to be identical to the results obtained with rituximab, taking into account that (even often repeated) retreatment in patients previously responding to rituximab is associated with a similar response in the majority of patients.1,24
Does a previous splenectomy impact on the probability of response to subsequent treatment with rituximab?
We compared the published response rates in patients given rituximab after failure of splenectomy to response rates after rituximab in patients with an intact spleen. Single case reports and studies with a mixture of ITP and AIHA cases or with a mixture of CAD and wAIHA cases that were analyzed as a single entity were excluded. In 44 patients given rituximab after failure of splenectomy, 22 (50%) achieved a CR, 12 (27%) a PR, and 10 (23%) failed.25,32,36,45,47-56 In 128 patients from the same studies, who were given rituximab and did not have a previous splenectomy, 82 (64%) achieved a CR, 30 (23.5%) a PR, and 16 (12.5%) failed. The difference is not significant (P = .17; χ2 test). With all the limitations of such an aggregate of heterogeneous studies, this suggests that the benefit from rituximab is not substantially compromised by a previous splenectomy. Conversely, the impact of previous treatment with rituximab on the success rate of splenectomy is unknown because of the very few reported cases.
Adverse effects from splenectomy and from rituximab
Splenectomy-related morbidity and mortality have been decreasing during the last decades, probably as a consequence of systematic preoperative vaccination, prevention of thrombosis, and widespread use of laparoscopic splenectomy. The incidence of postsplenectomy venous and arterial thrombotic events has recently been described in more detail. In the GIMEMA study, a thrombotic event was recorded in 11% of 308 patients with wAIHA and CAD, including 11 pulmonary embolisms and 13 deep vein thromboses.2 Thrombotic events were more frequent in patients who had undergone splenectomy (24% vs 8.7%). Surprisingly, the presence of a lupus anticoagulant or of anticardiolipin antibodies (present in 13% of patients) was not associated with an increased risk of thrombosis. In the same study, grade 3 pulmonary infections were associated with splenectomy but not with the number of lines of treatment or with the use of rituximab. Progressive multifocal leukoencephalopathy remains a major concern in patients given rituximab. In a series of 57 HIV-negative patients who developed progressive multifocal leukoencephalopathy after treatment with rituximab, 2 had been given rituximab to treat an autoimmune hemolysis associated with a lymphoproliferative disorder, including one with previous stem cell transplantation and a second one treated only with steroids and rituximab.57 Reactivation of hepatitis B infection should be prevented by antiviral prophylaxis.58
Overall, rituximab was recommended as a second-line therapy by the GIMEMA group.2 However, the decision remains a difficult one and is better made on the basis of individual considerations, taking into account operative risk, probability of thrombosis, availability of rituximab, and patient preferences.
What after rituximab failure?
Patients resistant to rituximab have been poorly studied. From the Belgian study, it can be suspected that resistance to rituximab is associated with a poor outcome: of 12 such patients with ITP, CAD, and wAIHA, 2 obtained a CR and 3 a PR with another treatment, 4 were left untreated, and 3 died of infectious complications.25 Prognosis after relapse from a previous successful treatment with rituximab is much better, as most relapsing patients will respond again to rituximab. Of 34 relapsing patients reported outside single case reports, 32 responded again to rituximab.26,32,36,37,54 Of note, reminiscent of thrombopoietin agonists in immune thrombocytopenic purpura, some patients seem to respond to erythropoietin. In the GIMEMA study, 13 of 14 patients with AIHA responded to treatment with erythropoietin but were given concomitant various therapies, which precludes any convincing conclusion.2
What can be concluded from the available evidence?
Rituximab has enlarged our therapeutic repertoire in AIHA. The most appropriate use of rituximab remains, however, to be defined, especially the dose, the timing of treatment, and its position compared with established treatments including splenectomy. The available data, although consistently reporting some efficacy of rituximab in wAIHA, are far from robust: practically all the published studies are retrospective, with poorly defined criteria of inclusion, heterogeneous criteria for response, admixture of primary and secondary cases, variable rituximab dose, and number of cycles. The Danish prospective study is a remarkable exception.46 The Danish investigators demonstrate that randomized studies aimed at defining the role of rituximab in wAIHA are feasible even in relatively small countries. However, at this time, such studies remain desperately few: 9 studies can be identified on clinicaltrials.gov with the use of “rituximab AND autoimmune hemolytic anemia” key words. Only 3 are randomized phase 3 studies, and none are open for recruitment.
Meanwhile, we cannot afford to ignore the use of rituximab in wAIHA and we need to rely on reasonable conclusions from available clinical data.
Obviously, upfront treatment with rituximab is reasonable in the rare patients with wAIHA secondary to a disorder requiring an anti-CD20 antibody, such as severe forms of low-grade lymphoma and chronic lymphocytic leukemia. Patients with a contraindication to steroids could also benefit from first-line rituximab. In patients relapsing after or refractory to steroids, splenectomy remains a valid option. However, rituximab is an alternative that seems to gain wider acceptance, especially in patients at high risk for surgery or who elect not to undergo surgery as illustrated by the increasing number of published patient series during the last years (Table 1). Response rates do not differ significantly from those observed after splenectomy but response duration may be shorter. However, several courses of rituximab can be administered with success after relapse.
More experience, especially longer follow-up after treatment with low-dose rituximab, is needed before it can be recommended in everyday practice. Maintenance with rituximab should be assessed in autoimmune disorders such as AIHA, especially in relapsing patients, because the underlying immunological disorder persists in most patients even after successful treatment, irrespective of its nature. Finally, as clinical trials are developing in the field of autoimmune hemolytic anemia, especially since the introduction of monoclonal antibodies, consensual definitions of responses to therapy should be implemented to allow valid comparisons between studies.
Authorship
Contribution: D.D., A.K., and A.D. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daan Dierickx, Department of Hematology, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium; e-mail: daan.dierickx@uzleuven.be.