In this issue of Blood, using a transgenic mouse model, Pickens et al describe that ectopic expression of ADAMTS13, exclusively in platelets, is able to provide effective protection in murine models of arterial thrombosis and thrombotic thrombocytopenic purpura (TTP).1
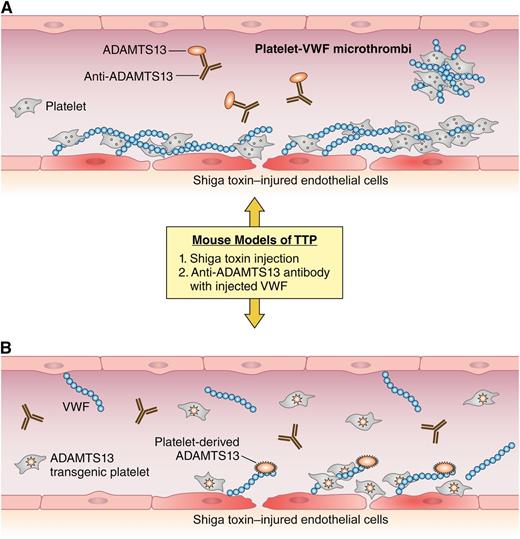
A simplified view of thrombotic challenges in the context of ADAMTS13 deficiency or platelet-expressed ADAMTS13. (A) In the blood vessel of an Adamts13-deficient mouse after Shiga toxin challenge, the damaged endothelium attracts the accumulation of platelets and VWF. In the absence of sufficient ADAMTS13 activity, platelets and VWF accumulate and form embolic microthrombi. The vessel of a wild-type mouse is shown with ADAMTS13 deficiency caused by anti-ADAMTS13 antibodies after injection of superphysiologic amounts of VWF. Here, any available ADAMTS13 is neutralized by injected antibody and VWF-rich emboli form. (B) The same prothrombotic challenges in a transgenic mouse showing recombinant ADAMTS13 expressed in platelets. Despite the 2 different prothrombotic challenges, the transgenic mouse is able to maintain anticoagulant ADAMTS13 activity and prevent the formation of VWF-rich microthrombi. Professional illustration by Patrick Lane, ScEYEnce Studios.
A simplified view of thrombotic challenges in the context of ADAMTS13 deficiency or platelet-expressed ADAMTS13. (A) In the blood vessel of an Adamts13-deficient mouse after Shiga toxin challenge, the damaged endothelium attracts the accumulation of platelets and VWF. In the absence of sufficient ADAMTS13 activity, platelets and VWF accumulate and form embolic microthrombi. The vessel of a wild-type mouse is shown with ADAMTS13 deficiency caused by anti-ADAMTS13 antibodies after injection of superphysiologic amounts of VWF. Here, any available ADAMTS13 is neutralized by injected antibody and VWF-rich emboli form. (B) The same prothrombotic challenges in a transgenic mouse showing recombinant ADAMTS13 expressed in platelets. Despite the 2 different prothrombotic challenges, the transgenic mouse is able to maintain anticoagulant ADAMTS13 activity and prevent the formation of VWF-rich microthrombi. Professional illustration by Patrick Lane, ScEYEnce Studios.
TTP is a life-threatening thrombotic microangiopathy that strikes individuals with deficiency of the von Willebrand factor (VWF) cleaving protease ADAMTS13. The most common form of TTP occurs in the presence of inhibitory autoantibodies against ADAMTS13, but it can also present in families in an autosomal recessive pattern, in which affected individuals have mutations in both alleles of ADAMTS13. The absence of sufficient ADAMTS13 activity leads to the accumulation of prothrombotic ultralarge VWF multimers, which, in the presence of other triggers, initiate the formation of VWF-rich (as opposed to fibrin-rich) thrombi in the microcirculation. Current treatment of acquired (autoantibody-mediated) TTP is plasma exchange, an intensive therapy that exposes patients to multiple donor blood products but is highly effective at reducing mortality from the disease. Case-dependent adjunct therapy is used in addition to plasma exchange and includes steroids, rituximab, immunosuppression, and, rarely, splenectomy. Therapies under development include bortezomib, N-acetylcysteine, recombinant ADAMTS13, and anti-VWF antibodies.2 The current study by Pickens et al is the first to suggest that transgenic platelets overexpressing ADAMTS13 may be a useful approach to deliver therapeutic recombinant ADAMTS13 to patients with acute TTP.
In a hemophilia A mouse model, other groups have previously demonstrated correction of this bleeding disorder when coagulation factor VIII is overexpressed in platelets, even in the presence of anti-factor VIII antibodies.3,4 More recently, lysozyme α-1-iduronidase was successfully expressed in platelets and demonstrated efficacy in a mouse model of Hurler syndrome.5 Now, in an initial step toward the development of platelet-delivered therapy for TTP, Pickens et al applied these techniques to address both congenital and acquired ADAMTS13 deficiency by generating transgenic mice that express recombinant human ADAMTS13 in megakaryocytes/platelets only.
To generate these mice, the authors inserted human ADAMTS13 complementary DNA into an expression vector under control of the megakaryocyte-specific murine glycoprotein 1B promoter; founder transgenic mice were subsequently bred onto an Adamts13 null background. The resulting transgenic mice expressed ADAMTS13 in platelets, and demonstrated only 0.5% activity in platelet-poor plasma compared with normal murine plasma, whereas robust activity was detected in transgenic platelet lysate. Human ADAMTS13 only partially colocalized with VWF and platelet factor 4 in transgenic megakaryocytes, suggesting additional localization of ADAMTS13 apart from platelet α granules, where its expression was expected. Transgenic mice had a statistically significant but mild increase in platelet count compared with strain-matched wild-type or ADAMTS13-deficient mice, but there was no difference in megakaryocyte ploidy or platelet aggregation.
Unlike many metalloproteases, ADAMTS13 does not require propeptide cleavage for activation. Regulation of its activity may be primarily allosteric and altered by plasma conditions and availability of its substrate, VWF.6 Although the intracellular location of ADAMTS13 in transgenic platelets remains to be clarified, the platelets do appear to release ADAMTS13 upon stimulation. As with patients with ADAMTS13 deficiency, mice expressing ADAMTS13 in platelets and Adamts13-deficient mice both demonstrated ultralarge VWF multimer patterns, suggesting that the majority of the ADAMTS13 activity in transgenic mice is available only upon platelet activation. When the authors challenged mice in an FeCl3 injury model,7 transgenic mice demonstrated thrombosis rates comparable to wild-type mice, suggesting that the prothrombotic roles played by VWF and platelets in this model can be effectively reduced by either plasma/endothelial- or platelet-derived ADAMTS13. The presence of ultralarge multimers in transgenic plasma and the performance of these mice in the FeCl3 model suggest that ADAMTS13 plays different functional roles depending on the specific cellular expression pattern. We can speculate that in humans, the baseline production of ADAMTS13 in hepatic stellate cells (or elsewhere) prevents ultralarge multimer accumulation, whereas local expression at sites of vascular injury prevents the development of pathologic thrombosis. In this model, transgenic platelets may be able to secrete much greater amounts of ADAMTS13 on demand than wild-type endothelium to prevent thrombosis accumulation locally but not enough ADAMTS13 systemically to prevent the accumulation of ultralarge multimers.
The figure depicts the performance of transgenic, wild-type and Adamts13-deficient mice in 2 models of TTP. Although not perfect replicates of TTP in humans, mouse models demonstrate thrombocytopenia, schistocytes, and VWF-rich thrombi that are also major features of the disease in humans.8,9 In the Shiga toxin model of TTP, the transgenic mice expressing ADAMTS13 in platelets demonstrated no mortality, thrombocytopenia, or detectable microthrombi, suggesting that platelet-delivered ADAMTS13, even in the absence of plasma or endothelial ADAMTS13, can protect against TTP. The authors also used an alternate model of TTP that involves injection of superphysiologic amounts of multimeric VWF into mice that are pretreated with an anti-ADAMTS13 antibody. In this model, the transgenic mice fared better than wild-type mice in the presence of the anti-ADAMTS13 scFv4-20, which may relate to the ability to outcompete antibodies at sites of platelet ADAMT13 activation/secretion. Regarding acquired TTP in humans, finding a “magic bullet” to reconstitute effective ADAMTS13 activity in the presence of circulating inhibitory antibodies remains a major therapeutic challenge. Therefore, the performance of the transgenic mice in the antibody injected model of TTP is of significant importance.
The development of platelet-delivered therapies may have advantages in several clinical situations complicated by the presence of a circulating inhibitor or where delivery of a deficient protein to the site of platelet accumulation would be therapeutic. Although very encouraging, these results are just the first hurdle on the long march toward platelet-delivered therapy in the clinic. Subsequent studies focusing on the generation of transfusion-ready platelets with adequate amounts of ADAMTS13 will be required. Additionally, investigators will have to determine if the therapeutic effects of ADAMTS13 delivery outweigh the increased rates of arterial thrombosis and mortality associated with platelet transfusions in patients with thrombotic microangiopathy.10
Conflict-of-interest disclosure: The author declares no competing financial interests.