Key Points
Human neutrophils use 2 independent mechanisms for the killing of unopsonized and serum-opsonized C albicans.
Unopsonized Candida killing depends on CR3 and CARD9 but not dectin-1; opsonized Candida killing on FcγR, PKC, and NADPH oxidase activity.
Abstract
Invasive fungal infections, accompanied by high rates of mortality, represent an increasing problem in medicine. Neutrophils are the major effector immune cells in fungal killing. Based on studies with neutrophils from patients with defined genetic defects, we provide evidence that human neutrophils use 2 distinct and independent phagolysosomal mechanisms to kill Candida albicans. The first mechanism for the killing of unopsonized C albicans was found to be dependent on complement receptor 3 (CR3) and the signaling proteins phosphatidylinositol-3-kinase and caspase recruitment domain-containing protein 9 (CARD9), but was independent of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity. The second mechanism for the killing of opsonized C albicans was strictly dependent on Fcγ receptors, protein kinase C (PKC), and reactive oxygen species production by the NADPH oxidase system. Each of the 2 pathways of Candida killing required Syk tyrosine kinase activity, but dectin-1 was dispensable for both of them. These data provide an explanation for the variable clinical presentation of fungal infection in patients suffering from different immune defects, including dectin-1 deficiency, CARD9 deficiency, or chronic granulomatous disease.
Introduction
The occurrence of fungal infections such as those with Candida and Aspergillus spp has progressively increased over the last decades as a consequence of the extensive use of immunosuppressive therapies.1,2 Invasive fungal infections, in particular, are associated with mortality rates of 40% to 50%.3,4
Neutrophils are the most important effector immune cells in fungal killing. It appears that adaptive immunity is primarily responsible for controlling Candida infection at the mucosal level by interleukin-17 (IL-17)–producing lymphocytes, whereas cells of the innate immune system are critical for preventing systemic candidiasis.5-8 The latter is illustrated by the increased prevalence of invasive fungal infections in patients with chemotherapy-induced neutropenia or with neutrophil functional defects, such as genetic deficiencies of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase as seen in chronic granulomatous disease (CGD) patients.9
The neutrophil mechanism of fungal killing is poorly understood. First, it is not known which pathogen recognition receptors and their downstream signaling pathways are involved in the killing of Candida albicans. Both C-type lectin receptors (CLRs; including dectin-1, dectin-2, and mincle, and also integrins, especially complement receptor 3 [CR3; CD11b/CD18, αMβ2]) have been demonstrated to recognize the β-glucans and mannans exposed on the C albicans cell wall.1,10,11 However, 2 studies performed with dectin-1 knockout mice contradict each other: 1 showed that dectin-1 was required for the control of systemic candidiasis, whereas the other claimed that this receptor was not relevant.12,13 The differential requirement of dectin-1 appeared to be Candida strain-specific.14 In humans, only 1 family with 3 dectin-1–deficient patients has been reported so far, showing clinically mucocutaneous fungal infections, but no invasive fungal infections.15 Moreover, as we have previously shown for human neutrophils, CR3 and not dectin-1 seems to be the major receptor for β-glucan recognition.16 Patients with a leukocyte adhesion defect (either LAD-I in which CD11/CD18 integrins are lacking or LAD-III [also known as LAD1/variant] syndrome with a deficiency of kindlin-3 required for leukocyte integrin activation) are genetically predisposed to invasive bacterial and fungal infections.17,18
In mouse models, both dectin-1 and CR3 have been described to trigger spleen-tyrosine kinase (Syk) and phosphatidylinositol-3-kinase (PI3K) to elicit a cytotoxic response and to activate the signaling adaptor protein CARD9 [caspase recruitment domain-containing protein 9].19-21 Recently, human patients with CARD9 deficiency have been characterized to suffer, typically, from Candida meningitis,22 and this was found to be associated with an impaired killing capacity of CARD9-deficient neutrophils toward Candida.23 Similarly, CGD patients with a defective NADPH oxidase system are also predisposed to invasive candidiasis, which may indicate a strict requirement to activate the NADPH oxidase system for microbial killing.9
In the present study, we demonstrate that human neutrophils use 2 distinct and independent mechanisms to kill C albicans, governed by the presence of opsonins on the yeast. The first mechanism, for the phagolysosomal killing of unopsonized C albicans, depends on CR3 recognition and signaling via Syk, PI3K, and CARD9, but is completely independent of NADPH oxidase activity. The second mechanism, for phagolysosomal killing of serum-opsonized C albicans, is strictly dependent on the Fcγ receptors, protein kinase C (PKC), and reactive oxygen species (ROS) production by the NADPH oxidase system, in which the tyrosine kinase Syk does have a role but PI3K does not. Further evidence for these distinct routes of fungal killing was obtained with cells from patients with well-characterized neutrophil dysfunctions. Our study clearly demonstrates the presence of 2 independent pathways for fungal killing that may contribute to the identification of novel therapeutic targets and provide an explanation for the clinical phenotype observed in dectin-1 deficiency, CARD9 deficiency, and CGD.
Materials and methods
Killing of microorganisms
Short-term microbicidal activity of granulocytes was determined as previously described.24 In brief, polymorphonuclear neutrophil (5.0 × 106 cells/mL) were cultured overnight with the C albicans–green fluorescent protein (GFP), at a yeast:neutrophil ratio of 4:1. The appearance of the hyphenated form of C albicans was assessed with a digital fluorescence microscope (Evos).
To determine the role of dectin-1 and CR3 in the C albicans killing, neutrophils were treated with a monoclonal antibody (mAb) against human dectin-1 (clone 259931; R&D systems), CD11b (clone 44A; ATCC), CD18 (clone IB4; ATCC), FcγRI (clone 10.1; BD Pharmingen), FcγRII (clone AT10; AbD Serotec), or FcγRIII (clone 3G8; BD Pharmingen). The inhibitors of Syk signaling were R406 (Selleckchem) and Bay 61-3606 (Sigma-Aldrich). The PI3K inhibitors were Wortmannin and LY294002 (Sigma-Aldrich). The PKC inhibitor was Go6983(Calbiochem).
Statistics
Statistical analysis was performed with GraphPad Prism version 5.00 for Windows (GraphPad Software). Data were evaluated by paired, the 2-tailed Student t test and by the Mann-Whitney test. The results are presented as the mean ± SEM. Data were considered significant when P < .05.
Study approval
The study was performed according to national regulations with respect to the use of human materials from healthy, anonymized volunteers with written informed consent, and all experiments were approved by the medical ethical committee of the Academic Medical Centre in Amsterdam according to the Declaration of Helsinki principles (version Seoul 2008).
Results
Unopsonized vs serum-opsonized Candida killing by human neutrophils
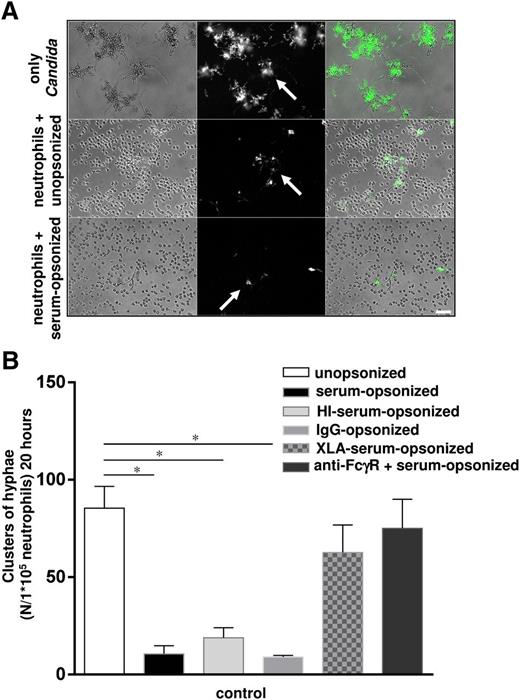
The pathogenicity of fungi is determined by their ability to germinate, invade tissues, and spread via the bloodstream.25 Although neutrophils are essential for antifungal host defense, the mechanism(s) for the killing of Candida have remained poorly defined, which led us to study Candida conidia killing and containment of hyphal growth by human neutrophils in more detail. We noticed that the capacity of human neutrophils from healthy controls to inhibit germination of C albicans into clusters of hyphae in an overnight assay, as well as to kill Candida conidia (2 hours), was strongly enhanced by opsonization with human serum (Figure 1A-B; supplemental Figure 1A, see supplemental Data available at the Blood Web site). The observed increase in Candida hyphae coincided with an increase in viability of Candida conidia after overnight incubation (supplemental Figure 1B). This enhancing effect of opsonization was completely dependent on immunoglobulin G (IgG) antibodies, as demonstrated as follows. First, we did not detect any significant reduction upon complement inactivation in the serum by prior heat treatment at 56°C for 30 minutes (Figure 1B and supplemental Figure 1). Second, IgG in immunoglobulin preparations for intravenous use (IVIG) in patients resulted in similar killing enhancement as observed with intact whole serum (Figure 1B and supplemental Figure 1). Third, the serum from an untreated X-linked agammaglobulinemia (XLA) patient who was completely lacking antibodies because of an inherited B-cell deficiency, did not enhance killing upon opsonization (Figure 1B). And, finally, mAbs against Fcγ receptors completely blocked the enhancement, supporting the notion that the serum opsonization of C albicans depends on the IgG directed against the candida cell wall (Figure 1B). Neutrophils treated with cytochalasin B to prevent actin polymerization for phagocytosis and particle uptake were impaired in inhibition of unopsonized and opsonized C albicans germination, which indicates that this process under these test conditions occurs through intracellular phagolysosomal killing (supplemental Figure 1C).
The killing of unopsonized and opsonized C albicans by neutrophils. (A) Neutrophils from healthy controls were cocultured overnight with unopsonized and serum-opsonized C albicans. White arrows indicate the observed clusters of GFP-positive C albicans hyphae. Scale bar is 50 μm. (B) Neutrophils from healthy controls were cocultured overnight with unopsonized, serum-opsonized, HI-serum-opsonized, XLA-serum-opsonized, IgG-opsonized C albicans–GFP or after blocking the FcγRs with mAbs (10 μg/mL) and the clusters of hyphae were assessed microscopically. Results are means ± SEM of at least 3 independent experiments. *P < .05. HI, heat inactivated.
The killing of unopsonized and opsonized C albicans by neutrophils. (A) Neutrophils from healthy controls were cocultured overnight with unopsonized and serum-opsonized C albicans. White arrows indicate the observed clusters of GFP-positive C albicans hyphae. Scale bar is 50 μm. (B) Neutrophils from healthy controls were cocultured overnight with unopsonized, serum-opsonized, HI-serum-opsonized, XLA-serum-opsonized, IgG-opsonized C albicans–GFP or after blocking the FcγRs with mAbs (10 μg/mL) and the clusters of hyphae were assessed microscopically. Results are means ± SEM of at least 3 independent experiments. *P < .05. HI, heat inactivated.
No role for dectin-1 (CLEC7A) in Candida killing by human neutrophils
With the understanding that serum opsonization was dependent on IgG, we subsequently focused on the surface molecules on human neutrophils involved in the uptake and killing of unopsonized C albicans conidia. The β-glucans form a major constituent of the C albicans cell wall. Dectin-1–deficient mice have been reported to suffer from Candida infections in experimental models.13 Dectin-1 on mice macrophages was shown to signal toward CARD9 to mediate NF-κB translocation and cytokine production, which is generally believed to contribute to survival from severe Candida infection.20
Because little is known about the role of dectin-1 in the neutrophil cytotoxic response, we first treated neutrophils of healthy controls with a blocking mAb against dectin-1. This mAb effectively blocked the binding of C albicans to dectin-1–transfected HEK cells (supplemental Figure 2).
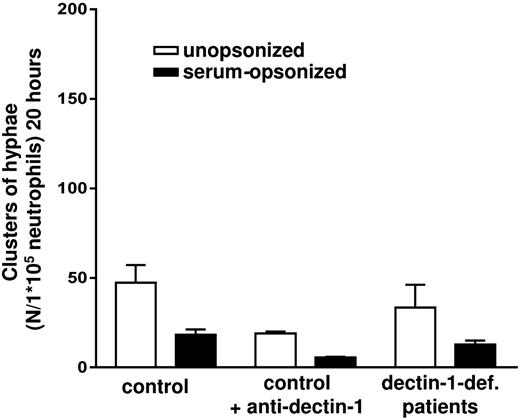
Upon treatment with this blocking dectin-1 antibody human neutrophils still effectively inhibited the germination of both unopsonized and opsonized C albicans conidia (Figure 2). Neutrophils treated with the antibody against dectin-1 also exhibited normal phagocytosis and killing of both the unopsonized and opsonized C albicans conidia (supplemental Figure 3A-B).
Dectin-1 is dispensable in the killing of C albicans. The number of C albicans–GFP hyphae clusters after coincubation with untreated neutrophils, treated with a blocking antibody against dectin-1 (20 μg/mL) and neutrophils from dectin-1–deficient patients A and B. Results are means ± SEM of at least 3 independent experiments.
Dectin-1 is dispensable in the killing of C albicans. The number of C albicans–GFP hyphae clusters after coincubation with untreated neutrophils, treated with a blocking antibody against dectin-1 (20 μg/mL) and neutrophils from dectin-1–deficient patients A and B. Results are means ± SEM of at least 3 independent experiments.
To confirm these findings, we subsequently tested the candidacidal neutrophil activity of previously identified patients with a homozygous stopcodon Tyr238X in the CLEC7A gene, leading to a partial deletion of the carbohydrate recognition domain of the dectin-1 receptor (supplemental Table 1, patients A and B).15 HEK cells expressing a dectin-1 receptor with this rare mutation did not bind C albicans, in contrast to cells expressing wild-type human dectin-1 (supplemental Figure 2). Remarkably, the dectin-1–deficient neutrophils from these patients were completely normal in the inhibition of both unopsonized and opsonized C albicans germination (Figure 2). The killing of unopsonized and opsonized C albicans conidia by the dectin-1–deficient neutrophils was also normal (supplemental Figure 3A). In addition, dectin-1 signaling was dispensable for the patients’ neutrophil capacity to generate hydrogen peroxide in response to zymosan or (un)opsonized C albicans (supplemental Figure 4). Also, the related CLRs dectin-2 and mincle are unlikely to be involved in neutrophil reactivity to C albicans because HEK cells transfected with either dectin-2 or mincle did not show Candida binding (supplemental Figure 2). Taken together, these findings demonstrate that human neutrophils are capable of phagocytizing and killing (un)opsonized C albicans through a dectin-1–independent pathway.
The lectin-binding role of CR3 in the killing of C albicans
We next examined the role of the integrin CR3 (CD11b/CD18, αMβ2) in the uptake and killing of C albicans. CR3 is highly expressed on neutrophils and has been reported to recognize β-glucans and mannans of the C albicans cell wall.1,11
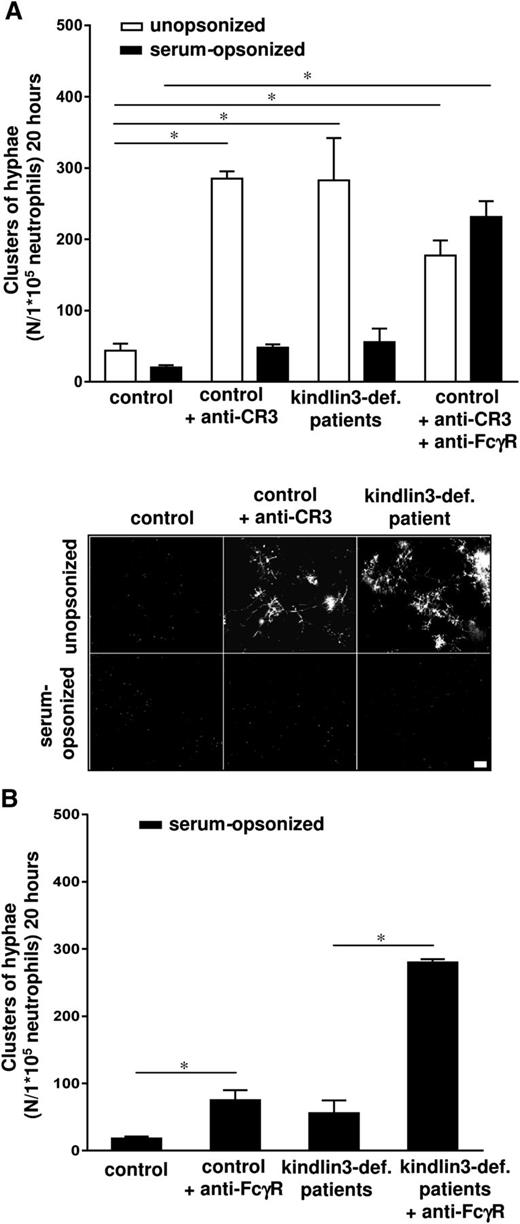
Neutrophils of healthy controls were treated with a blocking mAb against the common β2 integrin subunit (clone IB4) and one against the αM chain (clone 44A) of the integrin CR3. These cells showed a clear defect in the inhibition of unopsonized C albicans germination after overnight incubation (Figure 3A). This killing defect induced by CR3 blocking was prevented by prior opsonization of C albicans with normal human serum (Figure 3A). Treatment of neutrophils with blocking mAbs against CR3 and against the FcγRs resulted in an impaired killing of unopsonized and serum-opsonized C albicans (Figure 3A). Neutrophils incubated with blocking mAbs against CR3 displayed a slight reduction in early phagocytosis of C albicans conidia (supplemental Figure 3D).
The killing of unopsonized C albicans depends on CR3. (A) Neutrophils from healthy controls, treated with blocking antibodies against CR3 (10 μg/mL IB4 and 44A) and against FcγRs (10 μg/mL) and from kindlin3-deficient patients C and D were cocultured overnight with C albicans–GFP and the clusters of hyphae were assessed microscopically. Scale bar is 50 μm. (B) The inhibition of serum-opsonized C albicans germination by untreated and anti-FcγR (10 μg/mL) treated neutrophils from healthy controls and kindlin3-deficient patients. Results are means ± SEM of at least 3 independent experiments. *P < .05.
The killing of unopsonized C albicans depends on CR3. (A) Neutrophils from healthy controls, treated with blocking antibodies against CR3 (10 μg/mL IB4 and 44A) and against FcγRs (10 μg/mL) and from kindlin3-deficient patients C and D were cocultured overnight with C albicans–GFP and the clusters of hyphae were assessed microscopically. Scale bar is 50 μm. (B) The inhibition of serum-opsonized C albicans germination by untreated and anti-FcγR (10 μg/mL) treated neutrophils from healthy controls and kindlin3-deficient patients. Results are means ± SEM of at least 3 independent experiments. *P < .05.
For further confirmation of these findings in overnight germination, we tested neutrophils from patients with a FERMT3 mutation, causing the leukocyte adhesion defect in LAD-III (supplemental Table 1, patients C and D). In LAD-III, the hematopoietic protein kindlin3 is absent, resulting in a defect of the integrin CR3 and other leukocyte and platelet integrins to adopt a high-affinity ligand conformation.17,18,26 Kindlin3-deficient neutrophils were defective in the inhibition of unopsonized C albicans conidia germination while fully capable to inhibit serum-opsonized C albicans conidia germination in the same overnight killing assay (Figure 3A). Incubation of kindlin3-deficient neutrophils with blocking mAbs against FcγRs resulted in an increase in observed hyphae clusters of serum-opsonized C albicans as compared with untreated kindlin3-deficient neutrophils (Figure 3B). Because the uptake of Candida by the neutrophils is an essential aspect of the process of phagolysosomal killing, the immediate responses of the kindlin3-deficient neutrophils were studied and found to be impaired in the phagocytosis and subsequent early killing of unopsonized C albicans conidia, but not in case of IgG-opsonized C albicans conidia (supplemental Figure 3C-D).
Thus, the integrin CR3 has an essential role in the recognition, uptake, and killing of unopsonized C albicans conidia, which indicates that in human neutrophils not dectin-1, but the integrin CR3 controls the killing of unopsonized C albicans.
The killing of opsonized and not unopsonized C albicans depends on an intact NADPH oxidase system
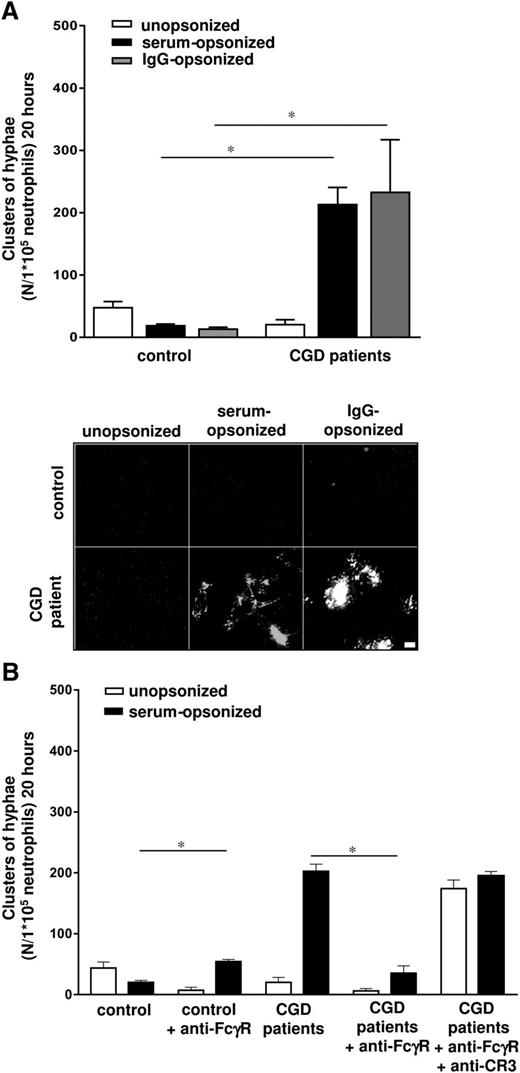
Because the lack of ROS formation by neutrophils upon addition of unopsonized zymosan is used as a diagnostic test for LAD-III18 and ROS production was also reduced in response to unopsonized Candida by kindlin3-deficient neutrophils (supplemental Figure 4), we investigated the role of the NADPH oxidase system in the killing mechanisms in greater detail. We studied patients with a complete defect in the NADPH oxidase activity, suffering from CGD. The CGD patients (supplemental Table 1, patients E-L) were deficient in either the cytosolic NADPH oxidase proteins, that is, p47phox or p67phox (N = 3 and N = 1, respectively) or the membrane-bound subunit (gp91phox; N = 4) of the NADPH oxidase system, resulting in a failure to produce ROS.27-29 To our surprise, the neutrophils of these patients inhibited the germination of unopsonized C albicans as efficiently as control neutrophils (Figure 4A), but were severely impaired in the killing of serum- or IgG-opsonized C albicans, resulting in massive outgrowth of hyphae in the overnight germination assay (Figure 4A). Incubation of CGD neutrophils with blocking mAbs against FcγRs resulted in normalization of the killing of serum-opsonized C albicans (Figure 4B). CGD neutrophils treated with blocking mAbs against the FcγRs and against CR3 were defective in the killing of both unopsonized and serum-opsonized C albicans (Figure 4B). In the short-term response, the CGD neutrophils showed a slightly reduced killing capacity of both unopsonized and serum-opsonized C albicans conidia compared with control neutrophils, despite normal phagocytosis (supplemental Figure 5A; data not shown). Only the defect in the inhibition of serum-opsonized C albicans germination was strikingly abnormal. Ultimately, the killing of unopsonized C albicans seems to be independent of ROS formation and can therefore not be held responsible for the defect in unopsonized Candida killing in case of a failure in CR3 function, as suggested above. These data together point toward distinct and independently activated routes of killing: one being activated by CR3-mediated uptake of unopsonized C albicans, and another being induced by IgG-FcγR–mediated uptake of serum-opsonized C albicans. These routes are activated by the surface receptors involved in recognition and uptake and dictate the subsequent killing mechanism involved. Only in the case of the latter route of IgG-mediated Candida uptake and not that of unopsonized Candida killing is the subsequent ROS formation by the NADPH oxidase activity essential for phagolysosomal killing.
CGD neutrophils fail to kill serum-opsonized C albicans. (A) Neutrophils from healthy controls and CGD patients E to L were cocultured overnight with C albicans–GFP, and the clusters of hyphae were assessed microscopically. Scale bar is 50 μm. (B) The inhibition of serum-opsonized C albicans germination by untreated, anti-FcγR (10 μg/mL) or anti-CR3/FcγR (10 μg/mL) treated neutrophils from healthy controls and CGD patients. Results are means ± SEM of at least 3 independent experiments. *P < .05.
CGD neutrophils fail to kill serum-opsonized C albicans. (A) Neutrophils from healthy controls and CGD patients E to L were cocultured overnight with C albicans–GFP, and the clusters of hyphae were assessed microscopically. Scale bar is 50 μm. (B) The inhibition of serum-opsonized C albicans germination by untreated, anti-FcγR (10 μg/mL) or anti-CR3/FcγR (10 μg/mL) treated neutrophils from healthy controls and CGD patients. Results are means ± SEM of at least 3 independent experiments. *P < .05.
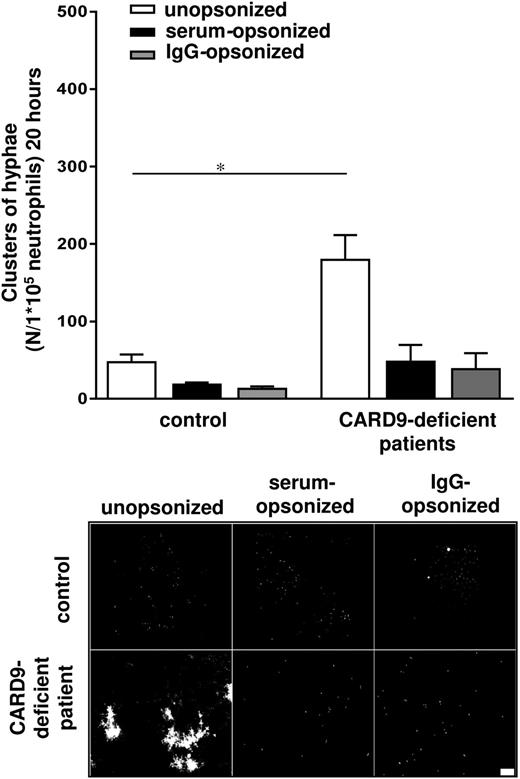
Selective role of CARD9 in the killing of C albicans
We have recently reported on the inability of neutrophils from a patient with a CARD9 deficiency to restrict the germination of Candida conidia, which may contribute to the associated invasive candidiasis observed in these patients23 (supplemental Table 1, patient M). In the current study, we confirmed this defect in a second, unrelated patient with CARD9 deficiency suffering from Candida meningitis (supplemental Table 1, patient N). The killing defect was overcome by opsonization of the C albicans conidia with human serum or IgG (Figure 5). As demonstrated, the short-term killing of unopsonized C albicans conidia by the CARD9-deficient neutrophils was already reduced (supplemental Figure 5B). The inability of CARD9-deficient neutrophils to control germination of unopsonized C albicans was not due to a defect in phagocytosis, as we already had excluded previously.23 Collectively, these data demonstrate that CARD9 is selectively required for the cytotoxic response of neutrophils against unopsonized C albicans but not against opsonized C albicans. Under both conditions, ROS generation was normal (supplemental Figure 4).
CARD9-deficient neutrophils show a selective C albicans killing defect. Neutrophils from healthy controls and CARD9-deficient patients M and N were cocultured overnight with C albicans–GFP and assessed microscopically. Scale bar is 50 μm. Results are means ± SEM of at least 3 independent experiments. *P < .05.
CARD9-deficient neutrophils show a selective C albicans killing defect. Neutrophils from healthy controls and CARD9-deficient patients M and N were cocultured overnight with C albicans–GFP and assessed microscopically. Scale bar is 50 μm. Results are means ± SEM of at least 3 independent experiments. *P < .05.
Thus, the recognition of either the unopsonized or the opsonized C albicans cell wall activates 2 distinct killing mechanisms by human neutrophils, that is, a CARD9- vs an NADPH oxidase–dependent pathway, respectively.
Signal transduction pathways in the killing of C albicans
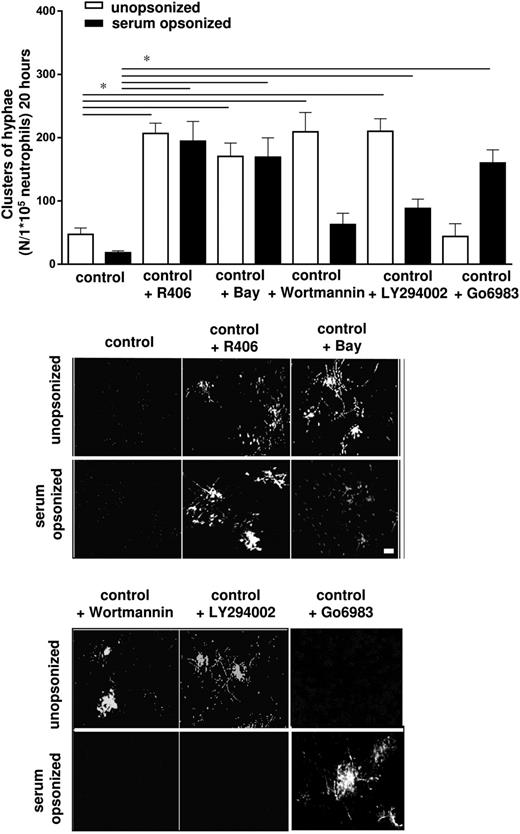
To confirm the notion of 2 distinct routes of killing that depend on the way how Candida is recognized and phagocytized, that is, either via CR3 or the FcγRs, we tested well-characterized compounds that are known to inhibit some key enzymes in major upstream signal transduction pathways.
Both the β2-integrin CR3 and the FcγRs have been described to signal through the tyrosine kinase Syk to elicit a cytotoxic response.19,21 To determine the role of Syk signaling in the killing of C albicans, we used 2 different pharmacological inhibitors of this kinase, that is, Bay61-3606 (2.5 µM) and R406 (2.5 µM). Neutrophils from healthy controls incubated with Bay or R406 were impaired in the inhibition of both the unopsonized as well as the opsonized C albicans germination as compared with untreated neutrophils (Figure 6). We investigated whether these effects were already explained by a defective early response. Indeed, treatment of neutrophils with Bay or R406 also decreased the short-term killing of unopsonized and opsonized C albicans conidia when compared with untreated neutrophils (supplemental Figure 6A). Whereas R406 or Bay reduced the phagocytosis of unopsonized C albicans for 50%, the same human neutrophils normally phagocytized serum-opsonized C albicans (supplemental Figure 6B).
Syk, PI3K, and PKC in the killing of C albicans. Untreated and treated neutrophils with inhibitors of Syk: R406 (2.5 μM) or Bay (2.5 μM), the PI3K inhibitors Wortmannin (100 nM), or LY294002 (10 μM) and the PKC inhibitor Go6983 (20 μM) were cocultured overnight with C albicans–GFP and clusters of hyphae were assessed microscopically. Scale bar is 50 μm. Results are means ± SEM of at least 3 independent experiments. *P < .05.
Syk, PI3K, and PKC in the killing of C albicans. Untreated and treated neutrophils with inhibitors of Syk: R406 (2.5 μM) or Bay (2.5 μM), the PI3K inhibitors Wortmannin (100 nM), or LY294002 (10 μM) and the PKC inhibitor Go6983 (20 μM) were cocultured overnight with C albicans–GFP and clusters of hyphae were assessed microscopically. Scale bar is 50 μm. Results are means ± SEM of at least 3 independent experiments. *P < .05.
To explore the signal transduction events downstream of Syk signaling, we studied the effect of PI3K inhibition by Wortmannin (100 nM) and LY294002 (10 µM). Neutrophils incubated with Wortmannin or LY294002 were found to be only inhibited in their capacity to prevent germination of unopsonized C albicans and not that of opsonized C albicans (Figure 6). PI3K inhibition by Wortmannin or LY294002 resulted in a decreased rate of phagocytosis and short-term killing of unopsonized C albicans, but a normal response to opsonized C albicans was found (supplemental Figure 6B). Whereas Syk was involved in the killing of unopsonized and opsonized C albicans, PI3K signaling seemed to be restricted to the killing of unopsonized C albicans in the CARD9 pathway, but not in the FcγR-mediated route to kill serum-opsonized C albicans by toxic ROS generation (Figure 7). To determine how the FcyRs activated the NADPH oxidase system we investigated PKC by the inhibitor Go6983 (20 µM). Neutrophils treated with the PKC inhibitor were impaired in the generation of ROS by the NADPH oxidase system but showed normal levels of Candida phagocytosis and proteolytic activity (supplemental Figure 6). The Go6983-treated neutrophils were not able to inhibit the germination of opsonized C albicans and normally inhibited the germination of unopsonized C albicans (Figure 6).
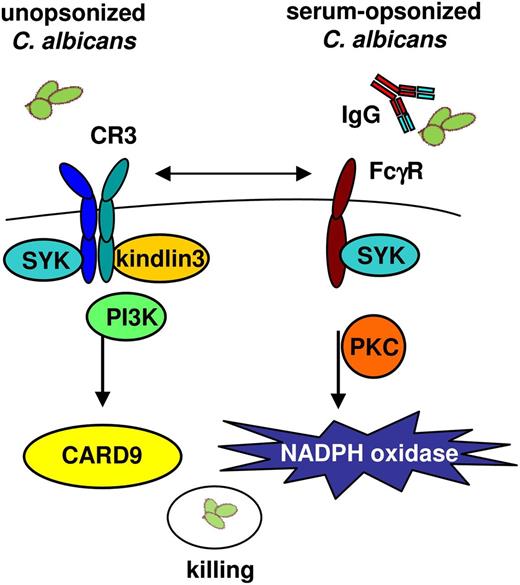
Neutrophil-mediated killing of C albicans. Two independent and distinct neutrophil-mediated killing mechanisms of C albicans were characterized. The killing of unopsonized C albicans depends on CR3 recognition, PI3K, and CARD9 signaling but is independent of NADPH oxidase activation. The killing of opsonized C albicans involved FcγRs, signaling through PKC and requires ROS formation by the NADPH oxidase system. Syk signaling is essential in both pathways for the killing of unopsonized and opsonized C albicans killing.
Neutrophil-mediated killing of C albicans. Two independent and distinct neutrophil-mediated killing mechanisms of C albicans were characterized. The killing of unopsonized C albicans depends on CR3 recognition, PI3K, and CARD9 signaling but is independent of NADPH oxidase activation. The killing of opsonized C albicans involved FcγRs, signaling through PKC and requires ROS formation by the NADPH oxidase system. Syk signaling is essential in both pathways for the killing of unopsonized and opsonized C albicans killing.
Discussion
The last 2 decades have witnessed a significant increase in the number of sepsis patients caused by fungal infections, due to the common use of immunosuppressive treatments or high-dosed chemotherapy-induced neutropenia.2-4
Neutrophils are the most numerous and potent effector cells in fungal killing, although the mechanism of killing is rather poorly understood. The aim of the current study was to investigate which surface receptors and signaling pathways are involved in the neutrophil cytotoxic response to C albicans. We identified 2 distinct and independent pathways for the killing of either unopsonized or IgG-opsonized C albicans. The first pathway for the phagolysosomal killing of unopsonized C albicans was dependent on CR3, Syk, PI3K, and CARD9 signaling, but independent of NADPH oxidase activity. The second pathway involved FcγRs, Syk, and ROS formation by the NADPH oxidase system and proved essential for the killing of serum-opsonized C albicans.
We excluded an imminent role of C-lectins, in particular dectin-1 (CLEC7A). Dectin-1 recognizes β-glucans of the C albicans cell wall and has been found essential in mouse models of Candida infection.1,12 In the present study, it was demonstrated that in human neutrophils dectin-1 is completely dispensable for the cytotoxic response to C albicans. As previously reported in β-glucan binding and the production of cytokines in response to C albicans,15 we could confirm that dectin-1–deficient monocytes were indeed impaired in the production of IL-6 in response to C albicans (supplemental Figure 7C). Impaired cytokine production by macrophages or dendritic cells may result in reduced numbers of Candida-specific Th17 cells, associated with mucocutaneous candidiasis,7,8 which is consistent with the observed clinical susceptibility to superficial C albicans infections in dectin-1–deficient individuals. Instead, human neutrophils use the lectin-binding integrin CR3 for Candida uptake and killing, in line with our previous observation that CR3 and not dectin-1 is the major receptor for β-glucan recognition by human neutrophils.16 In contrast to our findings, Li et al showed in a mouse model that dectin-1 is required to activate CR3 for the cytotoxic response to C albicans.30 Also, LAD-III patients with an activation defect of the integrins are predisposed to invasive fungal infections.17,26 Indeed, kindlin3-deficient neutrophils (as well as control neutrophils treated with blocking antibodies against CR3) were impaired in the capacity to inhibit germination of unopsonized C albicans resulting in massive outgrowth of hyphae, whereas kindlin3-deficient neutrophils showed a normal inhibition of serum-opsonized C albicans germination. Because the latter was IgG-mediated, treatment of these kindlin3-deficient neutrophils with antibodies against FcγRs resulted in an impaired inhibition of C albicans germination. Although deposition of complement fragment C3b has previously been indicated to facilitate phagocytosis of serum-opsonized Candida by CR3,31 our study supports the major role of FcγRs in the phagocytosis of serum-coated pathogens such as Candida.32 Whether the FcγRs inhibit the signaling through CR3 in case of serum-opsonized Candida or merely represent a stronger signal for killing cannot be deduced from our data.
In fact, upon investigation of the role of signaling pathways in the cytotoxic response to C albicans, we show that for human neutrophils both the integrin CR3- and the FcγR-mediated killing of unopsonized and opsonized C albicans, respectively, are Syk dependent. Studies with mice have shown that the integrin CR3 activates Syk to elicit antimicrobial responses such as ROS generation and the release of proteases in the phagolysosome.33 Syk is also involved in mice in FcγR-mediated phagocytosis of IgG-coated erythrocytes.34 Others have shown that β-glucan activates a CR3-Syk-PI3K pathway in neutrophils and thereby enhances tumor cell killing.21 In line with Honda et al, we found PI3K involved in superoxide production in response to platelet activating factor/fMLP and unopsonized C albicans but not in response to serum-opsonized C albicans (data not shown).35 Our results confirm the role of PI3K in neutrophils to be also critical in killing of unopsonized C albicans. Clearly, this CR3-Syk-PI3K pathway acts through the adaptor protein CARD9. In the present study, we extend our previous findings on CARD9 in the cytotoxic response to C albicans.23 In 2 unrelated CARD9-deficient patients suffering from Candida meningitis, the neutrophils were impaired in the killing of unopsonized but not of serum-opsonized C albicans. Wu et al demonstrated in mice that CARD9 is involved in bacteria-elicited production of ROS by bone marrow–derived macrophages and in in vivo host response.36 In contrast to these murine results, the CARD9-deficient neutrophils from 2 different patients showed a normal NADPH oxidase activity in response to various stimuli including Staphylococcus aureus. This could be explained by the fact that Wu et al used a different cell type and another Gram-positive bacterium, Listeria monocytogenes, or this simply illustrates the difference between CARD9-deficient mice and humans. To date, 4 reports have been published including 26 CARD9-deficient patients suffering from severe invasive fungal infections but none of them had severe bacterial infections.22,23,37,38 Moreover, we have previously shown that the neutrophil killing of S aureus by the patient cells was completely normal.23 These results suggest that nonoxidative killing mechanisms are important in the CARD9-mediated killing of unopsonized C albicans. It has been demonstrated in mice that a double knockout of elastase and cathepsin G results in susceptibility to invasive candidiasis.39 Future research needs to determine how exactly CARD9 regulates the nonoxidative killing of C albicans.
Because the predominant neutrophil antimicrobial activity is considered to depend on the formation of ROS by the NADPH oxidase system, we investigated the neutrophils from CGD patients with an inherited defect in ROS production. CGD neutrophils normally inhibited the germination of unopsonized C albicans but were severely impaired in the killing of opsonized C albicans. We also showed that after blocking FcγRs, the alternative pathway in CGD neutrophils induced the killing of serum-opsonized C albicans. Thus, the killing of opsonized C albicans depends on IgG recognition by FcγRs, signaling through PKC and the production of ROS by the NADPH oxidase system.40 The finding that CGD neutrophils fail to kill C albicans correspond with the increased prevalence of systemic Candida infections described in patients.9
Although suggestions that neutrophil extracellular traps of extruded DNA may have a role in the candidicidal activity,41 pretreatment of neutrophils with cytochalasin B showed that the inhibition of unopsonized and opsonized C albicans germination occurred through a phagolysosomal killing process.
Taken together, we characterized the presence of 2 distinct mechanisms used by the neutrophil in the host defense against C albicans. First, the killing of unopsonized C albicans depends on CR3 and the signaling proteins Syk, PI3K, and CARD9, but is independent of the NADPH oxidase system. The second mechanism used for the killing of opsonized C albicans depends on IgG recognition by FcγRs, signaling through Syk, PKC, and activation of the NADPH oxidase system (Figure 7). Apparently, the effects are binary in the sense that the neutrophil’s choice for 1 or the other killing mechanism depends on the initial step of C albicans recognition and uptake. There is cross-talk between the 2 pathways because binding of IgG-opsonized C albicans to FcγRs on the neutrophils inhibits induction of killing by the CR3-CARD9 pathway, which can be relieved by blocking the FcγRs. In addition, blocking of CR3 and the FcγRs on CGD neutrophils resulted in defective killing of both unopsonized and opsonized C albicans. These findings provide fundamentally new insights into the pathophysiological mechanism underlying immunological defects in the antifungal killing mechanisms of human neutrophils. Further unraveling the relevant molecular signaling pathways will help to identify novel targets for future antifungal therapeutics.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are very grateful to the patients and their parents for their cooperation.
R.P.G. was supported by the Landsteiner Foundation for Blood Transfusion Research (LSBR 1706) awarded to T.W.K.
Authorship
Contribution: T.W.K. is the principal investigators who conceived and designed the study.; R.P.G., J.L.v.H., A.T.J.T., M.v.H., and P.J.J.H.V. performed the experiments; F.L.v.d.V., M.H., and J.G.L. assisted in acquisition of the patient samples; T.K.v.d.B. contributed to the design of the study; D.R. helped with the interpretation and the presentation of the results; R.P.G. devised and performed the analyses and wrote the manuscript together with T.W.K.; and all authors approved the final manuscript revisions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roel P. Gazendam, Sanquin, Department of Blood Cell Research, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: r.gazendam@sanquin.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal