Key Points
Older age, insidious onset, no preceding infection, mild bleeding, and higher platelet count are the strongest risk factors for chronic ITP.
Intravenous immunoglobulin treatment seems to protect against development of chronic ITP.
Abstract
Childhood immune thrombocytopenia (ITP) is a rare autoimmune bleeding disorder. Most children recover within 6 to 12 months, but individual course is difficult to predict. We performed a systematic review and meta-analysis to identify predictors of chronic ITP. We found 1399 articles; after critical appraisal, 54 studies were included. The following predictors of chronic ITP in children, assessed in at least 3 studies, have been identified: female gender (odds ratio [OR] 1.17, 95% confidence interval [CI] 1.04-1.31), older age at presentation (age ≥11 years; OR 2.47, 95% CI 1.94-3.15), no preceding infection or vaccination (OR 3.08, 95 CI 2.19-4.32), insidious onset (OR 11.27, 95% CI 6.27-20.27), higher platelet counts at presentation (≥20 × 109/L: OR 2.15, 95% CI 1.63-2.83), presence of antinuclear antibodies (OR 2.87, 95% 1.57-5.24), and treatment with a combination of methylprednisolone and intravenous immunoglobulin (OR 2.67, 95% CI 1.44-4.96). Children with mucosal bleeding at diagnosis or treatment with intravenous immunoglobulin alone developed chronic ITP less often (OR 0.39, 95% CI 0.28-0.54 and OR 0.71, 95% CI 0.52-0.97, respectively). The protective effect of intravenous immunoglobulin is remarkable and needs confirmation in prospective randomized trials as well as future laboratory studies to elucidate the mechanism of this effect.
Introduction
Childhood immune thrombocytopenia (ITP) is a rare autoimmune disorder characterized by isolated thrombocytopenia (peripheral blood platelet count <100 × 109/L) in the absence of other causes that may be associated with thrombocytopenia.1 Most children present with a typical history of acute development of purpura and bruising, often after a mild viral infection. Management of newly diagnosed ITP consists of careful observation, regardless of platelet count. Severe bleeding, occurring in only 3% to 5% of children,2 requires treatment with corticosteroids, intravenous immunoglobulin (IVIg), or anti-Rhesus-D immunoglobulin, either alone or in combination and, if life threatening, also with platelet and red blood cell transfusions.3,4
Chronic ITP is currently defined as thrombocytopenia <100 × 109/L lasting for >12 months.1 About 20% to 25% of children with newly diagnosed ITP will develop chronic disease. Because of the high impact of ITP on a child’s everyday life and activities, as well as to decide whether treatment has to be instituted to influence the clinical course of ITP, it is of clinical significance if the course of the disease could be predicted at time of diagnosis.
Several clinical, therapeutic, laboratory, and genetic predictors of chronic ITP have been evaluated in children.5-7 A systematic review or meta-analysis, however, has not yet been performed. In this study, we systematically analyzed all informative studies related to the topic of childhood ITP, published from 1966 onwards and retrieved via a PubMed and EMBASE search, to determine the evidence for clinical, therapeutic, laboratory, and genetic predictors for development of chronic ITP in children.
Methods
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement (http://www.prisma-statement.org).8
Eligibility criteria
Types of studies.
We included observational cohort studies with newly diagnosed ITP patients studying the relationship among clinical, therapeutic, laboratory, or genetic parameters at time of diagnosis and development of chronic ITP published as an article or letter in a peer-reviewed journal in English, German, Dutch, or French, without publication-date restrictions.
Types of participants.
We included children (aged 3 months to 18 years) with newly diagnosed ITP.
Types of outcome measures.
The primary outcome is development of chronic ITP. Because the definition of chronic ITP has been changed recently, we used both the former definition of thrombocytopenia (<150 × 109/L lasting >6 months) and the new definition (platelet count <100 × 109/L lasting >12 months).1
Type of determinants.
We used all clinical, therapeutic, laboratory, or genetic parameters studied in patients with newly diagnosed ITP for its possible association with chronic disease.
Exclusion criteria.
(1) Study population <40 children (to avoid inclusion of studies with <10 children with chronic ITP); (2) no comparison between recovered and chronic ITP; (3) definitions of chronic ITP other than the formal old and new definition described above; (4) the predictors were not determined at time of diagnosis (except for genetic predictors); (5) the described therapy was not administered in the first days after presentation of symptoms; and (6) no full text available.
Information sources
Studies were identified by searching PubMed (1966 to present) and EMBASE (1980 to present) databases. The search was performed on May 2, 2012; afterward, additional studies were included by performing monthly searches in PubMed up to February 28, 2014. Of all articles taken for further assessment, bibliographic references were checked and citations were checked in Web of Science (1945 to present).
Search
We searched on domain (children AND ITP) combined with outcome (chronic) and on outcome combined with determinant (predictors OR therapy). The full search is listed in supplemental Table 1 (available on the Blood Web site). The search was designed and supervised by an experienced librarian and aimed at a high sensitivity.
Study selection
Two investigators (J.N. and K.M.J.H.-P.) independently screened all results by reviewing titles and abstracts. All potentially relevant studies were retrieved as full-text manuscripts. J.N. and K.M.J.H.-P. evaluated all studies for compliance with inclusion criteria. In case of any doubt for eligibility for inclusion, this was discussed with a third independent pediatric hematologist (M.C.A.B.). All included articles were critically appraised on their relevance and validity, and articles were excluded based on this appraisal (supplemental Table 2). An overview of our search and study selection is shown in the flowchart (Figure 1).
Data collection process
Duplicate reports of studies were excluded by checking authors’ names, authors’ affiliations, and titles. Duplicate inclusion of patients participating in >1 study was avoided by systematically evaluating patient recruitment periods and participating centers. Only if a different predictor was analyzed, a patient could be evaluated in >1 study.9-14
Data extraction and management
Data extraction from manuscripts was performed by 1 investigator (J.N.) and checked by 2 other investigators (K.M.J.H.-P. and C.W.B.B.). All data were entered in a structured electronic database. Data were only taken for further assessment if raw data for patients with recovered as well as chronic ITP were available. All authors of included articles were contacted to send additional data regarding possible predictors for chronic ITP to collect as many data as possible.
Data items
The following data were extracted from the included studies: first author, year of publication, study population, number of children with recovered and chronic ITP, country, type of study cohort, study period, prognostic determinants studied in children with recovered and chronic ITP, odds ratios (ORs) and their 95% confidence interval (95% CI), or P values and level of evidence based on the criteria of the Oxford Centre for Evidence-Based Medicine (CEBM) of 2011.
Risk of bias within individual studies
Risks of bias within individual studies are retrospective study design with an inherent risk of bias by the unblinded study investigators, missing data, bias in patient inclusion, or misinterpretations of clinical data and reports. To minimize publication bias of articles that only described significant or relevant data, we contacted all authors to send us additional data on all evaluated predictors of chronic ITP, including nonpublished data.
Summary measures
We calculated the summary weighted ORs and 95% CIs for all dichotomized predictors. For continuous variables, mean differences with 95% CI were calculated. To incorporate heterogeneity between studies, we used the random effects model by the Mantel-Haenszel method, using the Cochrane statistical package in RevMan (version 5.3). Differences were considered significant if the 95% CI did not contain 1.0 for dichotomous and 0.0 for continuous variables. Measures of heterogeneity were calculated and included in all forest plots created with RevMan.
Synthesis of results
Because of limited clinical and methodological heterogeneity, results of each individual study were pooled in a meta-analysis. Pooled ORs or means for developing chronic ITP for each prognostic determinant were calculated.
Risk of bias across studies
The outcome of studies may be related to the ethnical background of the patients, type of study centers (tertiary centers vs general hospitals), the use of either the former or new definition for chronic ITP, and differences in treatment strategy related to the time periods (treatment with steroids in earlier studies, IVIg and anti-Rhesus-D immunoglobulin in later studies, and, more recently, careful observation). Additional analyses as described below were performed to identify the risk of bias across studies.
Additional analyses
We evaluated whether small study data trends were present by arranging the studies in forest plots by study sample size and visually assessed asymmetry. In the same way, we evaluated the influence of the year of publication, definition of chronic ITP, hospital category, study category, and origin of the study population divided by continent. To verify whether the associations were influenced by the methodological quality of the studies, we repeated the calculations in a subgroup of studies that we considered to have a low chance of bias for this specific research question. In case of available complete sets of raw data, we planned to perform multivariate analyses.
Results
Study selection
A total of 1399 articles were retrieved by our search (Figure 1). After screening of title and abstract, 70 articles were critically appraised (see supplemental Table 2A). After screening all references, 1 additional study was found, because our search terms were not mentioned in the title or abstract of this study.15 Fifty-four articles were taken for further assessment based on our criteria for relevance and validity (see supplemental Table 2B).
Study characteristics
All studies were published between 1975 and 2013 and were performed in different continents. Most studies were single-center studies. Study populations varied between 43 and 1984 children and included children of all ages. Details of study characteristics are shown in supplemental Table 3.
Risk of bias within studies
We contacted all authors to collect additional data of every study. Unfortunately, only 4 authors16-19 would actually disclose their data. Within the data provided, no additional risk factors were found.
Synthesis of results
All ORs displayed in the results below refer to the odds of developing chronic ITP. All results for individual studies are summarized in supplemental Table 3.
Clinical predictors
Gender.
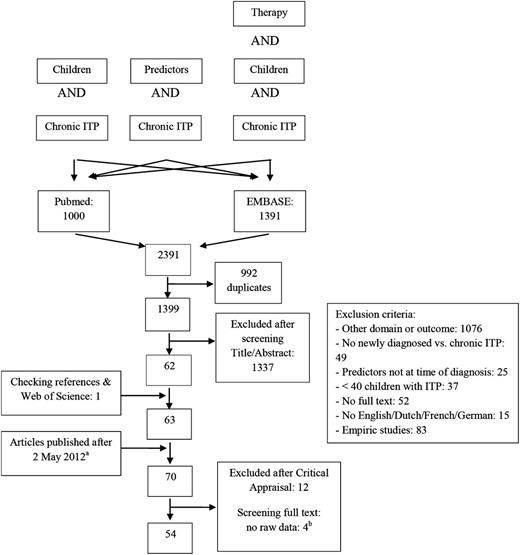
Twenty-five articles analyzed gender of ITP patients in relation to the course of disease.11,12,16,18-39 Results are displayed in Figure 2. Females developed chronic ITP significantly more often (OR 1.17, 95% CI 1.04-1.31).
Forest plot for female gender and age. ORs were calculated for chronic ITP; events = number of female patients or patients with age ≥11 years or ≥8 years. M-H, Mantel-Haenszel.
Forest plot for female gender and age. ORs were calculated for chronic ITP; events = number of female patients or patients with age ≥11 years or ≥8 years. M-H, Mantel-Haenszel.
Age.
Eighteen articles analyzed the mean age at time of diagnosis of ITP between children that recovered and those that developed chronic ITP.11,12,16,19,20,22-27,29,30,32,37,40-42 Two articles did not publish standard deviations or 95% CIs.30,40 Patients who developed chronic ITP were older, and the mean difference was 2.68 (95% CI 1.89-3.47) years. Age ≥8 years was studied in 4 studies with an overall OR for chronic ITP of 2.97 (95% CI 1.42-6.21) (see Figure 2).18,19,27,33 Thirteen articles studied age >11 years, with a statistically significant overall OR of 2.47 (95% CI 1.94-3.15; Figure 2).19-22,29,32-34,36,39,41,43,44
Preceding infections and vaccinations.
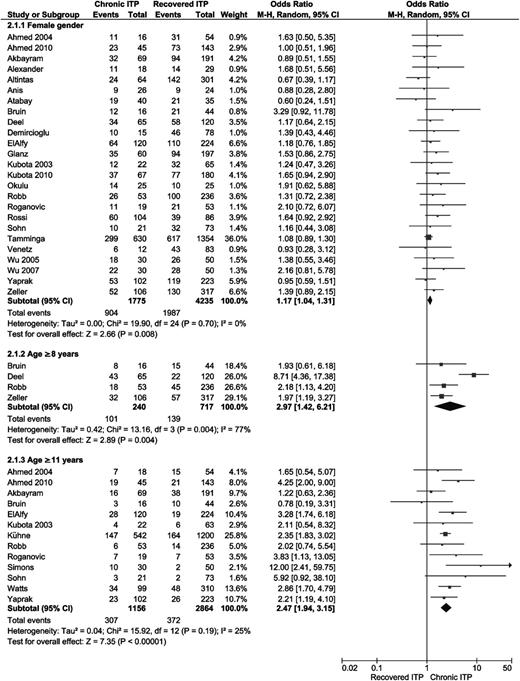
Seven studies analyzed whether an infection before the onset of ITP was correlated with development of chronic ITP19,22,23,29,30,32,37 ; 4 studies evaluated the occurrence of viral infections only.21,33,34,36 A preceding vaccination was evaluated in 3 studies,18,22,30 and 2 articles reported the incidence of a preceding infection or vaccination as one combined predictor.18,31 Figure 3 shows that the absence of an infection or vaccination in the medical history shortly before the first clinical signs of ITP is significantly correlated with a chronic course of the disease (pooled OR 3.08, 95% CI 2.19-4.32).
Forest plot for preceding infection and/or vaccination, an insidious onset, and season at time of diagnosis. Event = number of patients with no preceding infection and/or vaccination, with an insidious onset defined as symptoms for <14 days, or with diagnosis in the displayed season. M-H, Mantel-Haenszel.
Forest plot for preceding infection and/or vaccination, an insidious onset, and season at time of diagnosis. Event = number of patients with no preceding infection and/or vaccination, with an insidious onset defined as symptoms for <14 days, or with diagnosis in the displayed season. M-H, Mantel-Haenszel.
Onset of disease.
Bleeding symptoms.
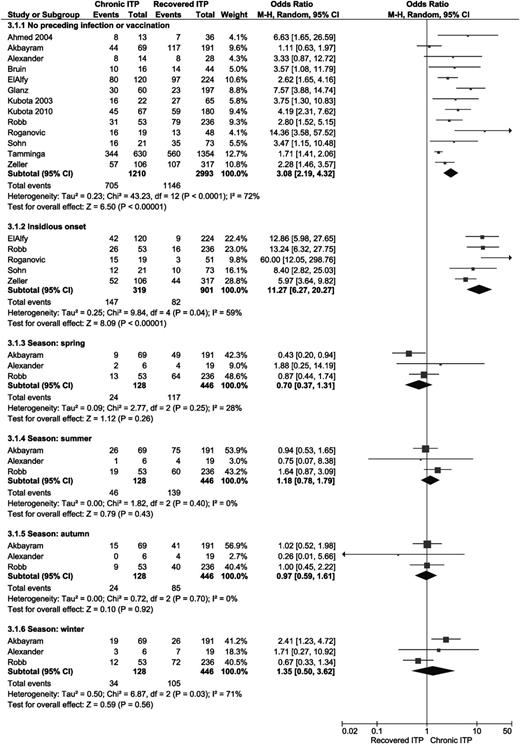
Two studies analyzed the correlation between the absence of bleeding symptoms at diagnosis and a chronic course of ITP, and both did not find this to be a risk factor.30,31 One study analyzed the presence of skin bleeding only.29 Four studies analyzed the presence of skin bleeding, regardless of other bleeding symptoms; also in this case, overall OR was not significant.18,22,30,31 Three studies found significant less chronic ITP in patients with mucosal bleeding (OR 0.39; 95% CI 0.28-0.54).29-31 Two studies separately analyzed epistaxis, gastrointestinal bleeding, oral mucosal bleeding, hematuria, and menorrhagia.22,29 In none of these subgroups was the overall OR significant. Three articles reported the grade of bleeding at time of diagnosis; no significant differences between recovered and chronic ITP patients were found.19,29,34 See Figure 4 for an overview of all results.
Forest plot for all bleeding symptoms and bleeding grades. ORs were calculated for chronic ITP; events = number of patients with type of bleeding or indicated bleeding grade at time of presentation. M-H, Mantel-Haenszel.
Forest plot for all bleeding symptoms and bleeding grades. ORs were calculated for chronic ITP; events = number of patients with type of bleeding or indicated bleeding grade at time of presentation. M-H, Mantel-Haenszel.
Remaining clinical predictors.
Three studies analyzed the season in which patients showed their first symptoms, but no significant correlation with chronic ITP was found (Figure 3).22,23,34 One study analyzed differences in incidence of chronic ITP in Vietnamese and German or Swiss children, but they did not find a significant difference.45
Therapeutic predictors
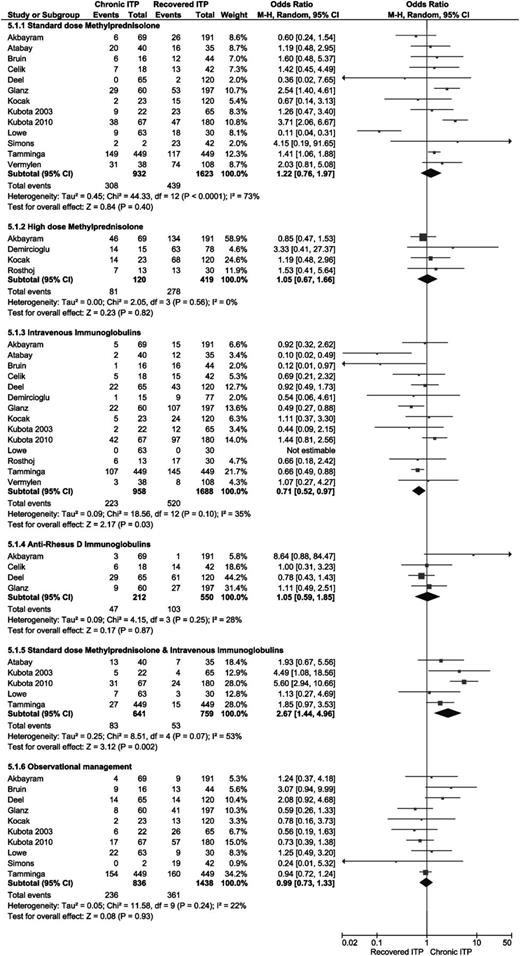
Four articles reported clinical course of ITP in relation with treatment at diagnosis with high-dose methylprednisolone (15-30 mg/kg per day),22,28,46,47 and 13 articles studied the effect of standard-dose methylprednisolone (SDMP; 1-2 mg/kg per day) (Figure 5).19,22,26,27,30-32,37,44,46,48-50 No significant differences were found. Fourteen studies analyzed the influence of treatment with IVIg and found significantly less chronic ITP in patients treated with IVIg (OR 0.71, 95% CI 0.52-0.97) (Figure 5).19,22,26-28,30-32,37,46-50 In 4 studies, the effect of treatment with anti-Rhesus-D immunoglobulin was analyzed (Figure 5), but no significant differences were found.22,27,30,49 Observational management vs any medical treatment was studied in 10 articles, but no significant differences were found (Figure 5).19,22,27,30-32,37,44,46,48 In 5 studies, combination therapy with SDMP and IVIg was analyzed.26,30,31,37,48 A significantly higher risk for chronic ITP was found in patients treated with a combination of IVIg and SDMP (Figure 5; OR 2.67, 95% CI 1.44-4.96).
Forest plot for all different therapies for ITP. ORs were calculated for chronic ITP; events = number of patients treated with the indicated therapy. M-H, Mantel-Haenszel.
Forest plot for all different therapies for ITP. ORs were calculated for chronic ITP; events = number of patients treated with the indicated therapy. M-H, Mantel-Haenszel.
Laboratory measurements
Platelet count.
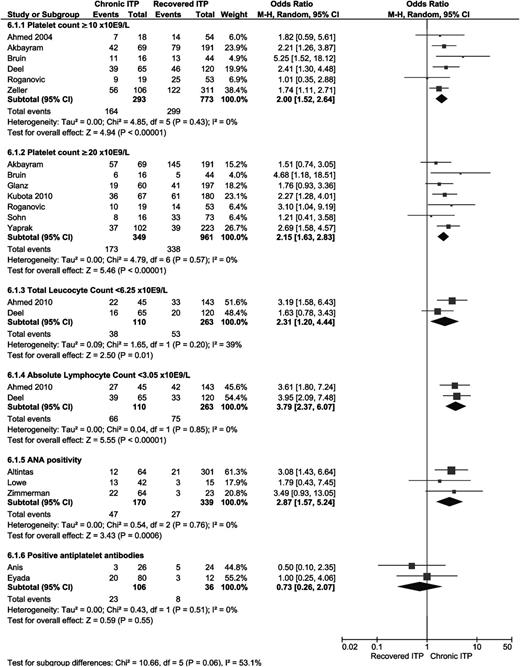
The mean platelet count at time of diagnosis was calculated in 12 studies.19,20,23,24,26,27,29,30,37,41-43 A significantly higher platelet count at diagnosis was found in patients who developed chronic ITP, with a mean difference 5.27 (95% CI 2.69-7.86). Four articles calculated a median platelet count, and 1 found significantly higher median platelet counts at diagnosis in patients who developed chronic ITP.16,21,28,48 Significantly more chronic ITP was found in children with a platelet count ≥10 × 109/L (6 studies; OR 2.00; 95% CI 1.52-2.64)18,19,21,22,27,34 and in children with a platelet count ≥20 × 109/L (7 studies; OR 2.15; 95% CI 1.63-2.83)19,22,30,31,34,36,39 at diagnosis (see Figure 6).
Forest plot for all laboratory measurements. ORs were calculated for chronic ITP; events = number of patients with laboratory results in accordance with the results displayed. M-H, Mantel-Haenszel.
Forest plot for all laboratory measurements. ORs were calculated for chronic ITP; events = number of patients with laboratory results in accordance with the results displayed. M-H, Mantel-Haenszel.
Mean platelet volume (MPV).
Two studies analyzed MPV at diagnosis of ITP. Both found a significant higher MPV in patients who developed chronic ITP. Yildirmak et al found a significantly higher mean MPV of 9.2 fL in patients developing chronic ITP compared with a mean of 8.1 fL in patients with recovered ITP (P = .04).42 Ahmed et al found significant more patients with an MPV >8.0 fL in the chronic ITP group (OR 15.4, 95% CI 4.1-56.9).21
Hemoglobin (Hb) levels.
White blood cells.
A mean total leukocyte count (TLC) was calculated in 4 articles, and 2 of these showed significantly lower TLCs in patients developing chronic ITP (mean difference −1.36, 95% CI −2.14 to −0.58).20,23,26,27 Two articles studied a TLC <6.25 × 109/L as a predictor for chronic ITP. The combined OR of both studies showed a significant OR of 2.31 (95% CI 1.20-4.44) (see Figure 6).20,27 The investigators also calculated the mean absolute lymphocyte count (ALC) and the OR of an ALC <3.05 × 109/L. For both parameters, they found a significantly lower ALC in patients developing chronic ITP (mean difference −1.56, 95% CI −2.04 to −1.08; total OR 3.79, 95% CI 2.37-6.07). The mean lymphocyte percentage was studied in 1 study, and this showed a significant difference (P = .006) as well.20
Anti-nuclear antibody (ANA) positivity (>1:80).
Platelet antibodies.
Two studies analyzed the presence of platelet antibodies, and both did not find a significant difference (Figure 6).25,52 Nielsen et al analyzed the presence of anti-glycoprotein antibodies and found significant less frequent anti-glycoprotein antibodies in patients who developed chronic disease (OR 0.11, 95% 0.01-0.84).53 Yildirmak et al studied the percentage of platelet-associated immunoglobulin M and immunoglobulin G and found a significantly lower percentage of platelet-associated immunoglobulin M in patients who developed chronic ITP.42 They also analyzed the mean percentage of platelet surface antigens but found no significant differences.
Bone marrow parameters.
Alexander et al found no differences in the amount of megakaryocytes in bone marrow but did find significantly higher percentages of eosinophilic granulocytes in bone marrow of patients developing chronic ITP.23 Ding et al reported a higher presence of human cytomegalovirus in bone marrow of patients developing chronic ITP (tested by Wright stain) (OR 10.68, 95% CI 1.31-86.66).54
Remaining laboratory measurements.
Genetic factors
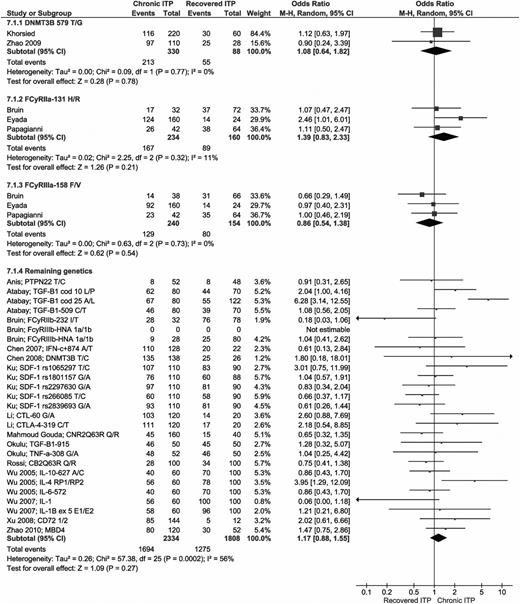
A total of 18 articles analyzed the allele frequencies of 29 different genes in patients who recovered and those with chronic ITP.9-16,19,25,26,35,52,55-59 Three polymorphisms (DNMT3B-579 T/G, FcyRIIa-131 H/R, and FcyRIIIa-158 F/V) were analyzed in >1 study and did not show any significant differences. Of all other polymorphisms, analyzed in single studies only, 2 allele frequencies showed significantly higher risks for developing chronic ITP: TGF-B1 cod 25 allele A (OR 6.28; 95% CI 3.14-12.55) and IL-4 intron 3 allele RP1 (OR 3.95; 95% CI 1.29-12.09).11,26 All genetic studies are summarized in Figure 7.
Forest plot for all genetic predictors. ORs were calculated for chronic ITP; events = the allele frequency of the first mentioned allele (for example allele T in DNMT3B 579 T/G); total = total allele frequency for both alleles, thus double the number with respect to the number of patients. M-H, Mantel-Haenszel.
Forest plot for all genetic predictors. ORs were calculated for chronic ITP; events = the allele frequency of the first mentioned allele (for example allele T in DNMT3B 579 T/G); total = total allele frequency for both alleles, thus double the number with respect to the number of patients. M-H, Mantel-Haenszel.
Additional analyses
To check whether there was a risk of bias across studies, we performed data-trend analysis by visually assessing forest plots. No data trends were found for study size, year of publication, definition of chronic ITP, hospital category, study category, and origin of the study population divided by continent (data not shown). No multivariate analyses could be performed due to the unavailability of complete sets of raw data.
Discussion
Childhood ITP is considered to be a heterogenous disease regarding both pathophysiology and clinical course.60,61 Literature provides little evidence for clinical decision making to find the right treatment strategy for individual patients.62 In this study, we have, for the first time, systematically reviewed and analyzed all clinical, laboratory, therapeutic, and genetic predictors for the outcome of childhood ITP.
Among treating physicians, patients, and parents, there is a great need for reliable predictors for the outcome of childhood ITP at the time of diagnosis as well as after initial therapy. This would help clinicians to provide patients and their parents with specific information about the expected clinical course, which may help to minimize anxiety and the impact of the disease on daily life. Furthermore, it could guide the decision on therapeutic management of the disease, especially if treatment would prevent development of chronic disease.
Several authors have tried to identify these predictors. The Nordic Society of Pediatric Haematology and Oncology (NOPHO) ITP Working group developed a predicting score for prolonged duration of ITP, based on their Nordic ITP study data, that contains the age at onset of disease, duration of symptoms, preceding infection, gender, platelet count at diagnosis, and the presence of wet purpura.63 Donato et al validated this score in a cohort of children presenting with ITP in Argentina.64 Revel-Vilk at al published a simple prediction score based on retrospective data of 472 children that included age and duration of bleeding symptoms at diagnosis.65 Finally, Yacobovich et al recently published a narrative review of several clinical, therapeutic, and genetic predictors for recovery of childhood ITP.66 However, neither of these studies performed an extensive literature search, and results were not systematically reviewed.
The results of our systematic review and meta-analysis show that predictors that were thought to be related to the course of childhood ITP, like age, gender, preceding infection, duration of symptoms, bleeding tendency, and platelet count at diagnosis, are indeed supported by a considerable level of evidence. Furthermore, our review adds some new possible predictors, like leukocyte count, ANA positivity, and treatment with a combination of corticosteroids and IVIg.
Remarkable is the apparent protective effect of IVIg treatment against development of chronic disease. The question is whether this is a true effect of IVIg or is based on confounding factors. It might be that IVIg has beneficial long-term immunomodulatory effects (eg, downregulating of the autoimmune response).67 In other human autoimmune diseases and in a mouse model of autoimmune ITP, it has been shown that IVIg causes an increase in the number and function of regulatory T cells (Tregs).68-71 Tregs contribute to the maintenance of peripheral immune tolerance.72 Several studies have shown lower frequencies and/or impaired function of Tregs in adults as well as in children with ITP.73-77 Although the exact mode of action remains unclear, IVIg treatment might restore the immunologic balance by inducing Tregs and so might influence the clinical course of the disease. In children with lower platelet counts and higher bleeding tendency, both associated with a lower rate of chronic disease, treatment will be instituted more often than in children with higher platelet counts or a low bleeding tendency. On the other hand, the higher rate of chronic disease in children who received a combination of steroids and IVIg seems to contradict this explanation, because the regular reason to give this combination is severe bleeding with low platelet counts as well. Based on the OR of 0.71 and an estimated 25% incidence of chronic disease, the number needed to treat with IVIg to prevent 1 child with chronic disease would be 14. However, at this moment, there are no large studies available reporting the incidence of chronic disease in children who only received observation, so an exact number to treat cannot be calculated. Future prospective studies, preferably randomized and combined with laboratory studies, are needed to find out if IVIg really protects against the development of chronic ITP and, if so, by what mechanism this effect is caused and if treating all children with IVIg would be cost effective, clinically effective, and tolerable.
Of course, our systematic review and meta-analysis has some limitations. First of all, some studies used different definitions or intervals for several predictors. Only if data for recovered as well as chronic patients were provided could ORs could be calculated. Some possible useful articles had to be excluded for this reason. Despite our effort to retrieve additional data from all authors in order to obviate this, we received these data from only 4 authors.16-19 Because of the recently changed definition of chronic ITP, fewer children are categorized as having chronic ITP in studies using the new definition. Hence, the outcome between the studies we analyzed could be different. In 8 studies, the new definition of chronic ITP was used.25,27,49,52,55,56 However, assessment of forest plots comparing results of studies using the old definition vs the new definition for chronic ITP did not show any differences (data not shown).
Due to the lack of raw data, we could only perform univariate analyses. Several predictors might be associated, for example, lower platelet counts and more severe bleeding symptoms as well as bleeding severity and the choice of medical treatment. A multivariate analysis could have taken these associations into account and could identify independent, objective and robust predictors. To perform such an analysis, large numbers of cases as well as identical diagnostic and therapeutic protocols to exclude selection bias are needed, which would only be feasible in collaborative study groups, like the Intercontinental Cooperative ITP Study (ICIS) group.
In conclusion, by performing this systematic review and meta-analysis, the following predictors of chronic ITP in children, assessed in at least 3 studies, have been identified: female gender (OR 1.17, 95% CI 1.04-1.31), older age at presentation (age ≥11 years; OR 2.47, 95% CI 1.94-3.15), absence of preceding infection or vaccination (OR 3.08, 95 CI 2.19-4.32), insidious onset (OR 11.27, 95% CI 6.27-20.27), higher platelet counts at presentation (≥20 × 109/L; OR 2.15, 95% CI 1.63-2.83), positive ANA titers (OR 2.87, 95% 1.57-5.24), and treatment with a combination of SDMP and IVIg (OR 2.67, 95% CI 1.44-4.96). Mucosal bleeding at diagnosis (OR 0.39; 95% CI 0.28-0.54) and treatment with IVIg (OR 0.71, 95% CI 0.52-0.97) seem to protect against development of chronic disease.
Recommendations
The information provided by our systematic review serves the need for identification of reliable predictors for the outcome of ITP and will guide researchers in the field of pediatric as well as adult ITP to reflect on their own research as well as to design future studies. The possible protective effect of IVIg treatment needs confirmation in prospective randomized studies. Based on our review, new prediction scores can be generated and tested in large cohorts of patients. To be able to compare prognostic risk factors, in future studies, all predictors should be strictly defined and be recorded at time of diagnosis of ITP. In this way, we might be able to identify patients at higher risk of developing chronic ITP who may benefit from treatment to prevent a chronic course of ITP.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mrs B. M. R. Kramer, librarian in the University Medical Center Utrecht, for her assistance with performing the literature search.
Authorship
Contribution: K.M.J.H.-P. designed the study, coordinated and supervised data collection, carried out analyses, interpreted data, drafted the initial manuscript, and approved the final manuscript as submitted; J.N. collected data, carried out analyses, interpreted data, drafted the initial manuscript, and approved the final manuscript as submitted; C.W.B.B. carried out analyses, interpreted data, reviewed and revised the manuscript, and approved the final manuscript as submitted; M.d.H. designed the study, reviewed and revised the manuscript, and approved the final manuscript as submitted; and M.C.A.B. designed the study, coordinated and supervised data collection, carried out analyses, interpreted data, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katja M. J. Heitink-Pollé, University Medical Center Utrecht/Wilhelmina Children’s Hospital, Department of Pediatric Hematology-Oncology, Room Number KC 03.063.0, Postbox 85090, 3508 AB Utrecht, The Netherlands; e-mail: k.m.j.heitink-polle@umcutrecht.nl.
References
Author notes
K.M.J.H.-P. and J.N. contributed equally to this study.