Key Points
Tolerance of rituximab is acceptable in ITP, and with its benefit/risk ratio may remain a valid option for treating ITP in adults.
Abstract
We conducted a prospective multicenter registry of 248 adult patients with immune thrombocytopenia (ITP) treated with rituximab to assess safety. We also assessed response and predictive factors of sustained response. In total, 173 patients received 4 infusions of 375 mg/m2 and 72 received 2 fixed 1-g infusions 2 weeks apart. The choice of the rituximab regimen was based on the physician’s preference and not patient characteristics. Overall, 38 patients showed minor intolerance to rituximab infusions; infusions had to be stopped for only 3 patients. Seven showed infection (n = 11 cases), with an incidence of 2.3 infections/100 patient-years. Three patients died of infection 12 to 14 months after rituximab infusions, but the role of rituximab was questionable. In total, 152 patients (61%) showed an overall initial response (platelet count ≥30 × 109/L and ≥2 baseline value). At a median follow-up of 24 months, 96 patients (39%) showed a lasting response. On multivariate analysis, the probability of sustained response at 1 year was significantly associated with ITP duration <1 year (P = .02) and previous transient complete response to corticosteroids (P = .05). The pattern of response was similar with the 2 rituximab regimens. With its benefit/risk ratio, rituximab used off-label may remain a valid option for treating persistent or chronic ITP in adults. This trial was registered at www.clinicaltrials.gov as #NC1101295.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disease characterized by low platelet count primarily from platelet autoantibodies that mediate platelet destruction in the reticuloendothelial system.1,2 These autoantibodies can also inhibit megacaryocyte proliferation and maturation with impaired platelet production. The major role of B cells is to produce antibodies. Thus, B cells play an important role in the pathophysiologic aspects of ITP; a rational approach to ITP treatment involves B-cell depletion with rituximab.3,4
It has been more than a decade since the first reports of rituximab use for ITP.5 Although results of only a few randomized studies have been published, rituximab is still largely used off-label as second- or third-line treatment in many countries, depending on its availability.6,7 It is considered a valid option for managing persistent or chronic ITP but the respective place of rituximab, splenectomy, and thrombopoietin agonists is debated for ITP patients with failure to achieve long-term remission with corticosteroids.8-10 Although the short-term efficacy of rituximab is now established, several important questions remain.
Assessing the tolerance and particularly risk of severe infection with rituximab for ITP is crucial.6-8 Rituximab was initially developed to treat lymphoma, and tolerance in this setting has been considered acceptable but may be overestimated. The risk of infection was initially a matter of concern and the use of rituximab has been associated with several severe and sometimes fatal infections, including cytomegalovirus, hepatitis B reactivation, Pneumocystis jiroveci pneumonia, parvovirus B19 infection, and progressive multifocal leukoencephalopathy (PML) caused by reactivation of the John Cunningham polyoma virus.11-13 Thus, some physicians and patients are reluctant to use the agent.6,8 In an extensive review of the literature, Arnold et al14 reported poor tolerance of rituximab in ITP, with a death rate of 2.9% and several deaths related to infection. However, the role of rituximab in these infectious complications is not always clear, because some patients received multiple prior immunosuppressive and/or cytotoxic treatments. The safety of rituximab has been better studied in other autoimmune diseases, and a large prospective registry of patients receiving rituximab for rheumatoid arthritis (RA) reported a rate of severe infection of 5 per 100 patient-years.15 No such study exists for ITP, and the safety profile of the drug in clinical practice is not clearly established.
Another important question is the efficacy of rituximab, and little is known about the predictive factors of sustained response.4,16 Young age, female gender, short duration of disease, achievement of complete response, and few previous treatments may be associated with sustained response. However, conflicting results have been reported, and we lack a clearly identifiable predictor of response to treatment with rituximab.
The last important issue is the best therapeutic rituximab regimen for ITP. The standard “lymphoma-like regimen” (ie, 4 weekly doses of 375 mg/m2) was usually used in most studies and is used in clinical practice.7,9 Some groups from the United Kingdom and Italy reported the efficacy and safety of rituximab with 4 weekly infusions of a fixed 100-mg dose but with a short duration of response.17,18 Recently, our retrospective study suggested that 2 fixed 1-g doses on days 1 and 15 as is used for other autoimmune diseases could be as effective as the standard regimen.19
Four years ago, we started a noninterventional prospective registry of adult patients with ITP in France (the “ITP-ritux registry”; www.clinicaltrials.gov #NC1101295) to investigate the safety (primary outcome) and efficacy of rituximab outside of a clinical trial. During 2 years, 255 patients were included. A follow-up duration of 5 years is planned. We report here the first results after a median follow-up of 2 years for the entire cohort.
Patients and methods
Organization of the ITP-ritux registry
The ITP-ritux registry is an ongoing nationwide prospective cohort study investigating the long-term safety and efficacy of rituximab for adult ITP. A population of 250 patients was chosen to allow for estimating the safety of rituximab with acceptable precision. The range of precision expected was from <2% to 6% for rates of adverse events ranging from 2% to 50% with a 5% α risk. The registry was started in July 2010 and closed in July 2012 after the inclusion of 255 patients. It was established by the French referral center for adult immune cytopenias, which includes a national network of 37 departments with ITP experts; 31 centers agreed to participate.
All consecutive patients ≥18 years of age with a diagnosis of primary ITP followed in the centers participating to the study and who received rituximab during the period of the study were prospectively included. No patients denied participation. The diagnosis of primary ITP was based on criteria established by the international working group on thrombocytopenia and French guidelines.9,20,21 Briefly, ITP diagnosis was mainly based on basic evaluation of the patient and family history, normal physical examination except bleeding signs, complete blood count and reticulocyte count, and peripheral blood smear analysis. Bone marrow examination was recommended only for patients older than 60 years and those with systemic symptoms and physical or biologically abnormal signs. Systematic serologic evaluation of HIV and hepatitis C infection was recommended with antinuclear antibody testing. Patients with secondary ITP were excluded except for those positive for antinuclear antibodies but without overt systemic lupus erythematosus. Patients who previously received rituximab were also excluded.
In France, ITP was treated in hematology or internal medicine departments. In the departments of internal medicine, staff are more familiar with the more convenient “RA-like” rituximab regimen (2 fixed 1-g infusions on days 1 and 15) used to treat other autoimmune diseases such as RA or systemic lupus erythematosus rather than the “standard regimen” (4 weekly 375-mg/m2 infusions) used to treat lymphoma. Because our study was observational and noninterventional, the choice of rituximab regimen was based on the physician’s preference and not patient characteristics.
Before each rituximab infusion, the recommendation was to premedicate with 1 g paracetamol with infusion of 100 mg diphenhydramine chlorhydrate and 100 mg methylprednisolone.
Response to treatment was assessed according to international guidelines: complete response, platelet count >100 × 109/L; and response, platelet count 30 to 100 × 109/L with at least a doubling of the pretreatment count.20 To manage ITP during follow-up, patients requiring another treatment, including a new rituximab cycle, were considered nonresponders, regardless of platelet count: only rescue therapy with a short course of corticosteroids or intravenous immunoglobulin infusions administered <8 weeks after the date of the first rituximab infusion was authorized.
The phases of ITP were defined according to international guidelines: “newly diagnosed ITP” was used for all cases at diagnosis, “persistent ITP” for between 3 and 12 months from diagnosis, and “chronic ITP” for ITP lasting longer than 12 months.20 This study was approved by the French authorities and by the Committee for Personal Protection of Henri Mondor, Créteil. Research was conducted in accordance with the Declaration of Helsinki.
To participate in the registry, investigators had to indicate their willingness to follow their patients prospectively for 5 years. According to the noninterventional observational design of the study, no laboratory test was specifically required by the protocol at the time of ITP diagnosis, at inclusion, or during follow-up. Results of all ITP-related tests ordered by the clinician at baseline or during follow-up were recorded on an electronic case report form (eCRF).
Data were collected at baseline by the clinician (at the time of the patient’s first exposure to the drug) and at 1-, 3-, and 6-month follow-up visits, then every 6 months or at disease relapse. If a patient had no follow-up visit for more than 10 months, the primary care physician or hospital physician was contacted. Three research study nurses were specifically trained in features of ITP, rituximab use, and the completion of the eCRF by the 2 coordinators of the study (M.K. and B.G.). Study nurses visited each participating center regularly to update the clinical and biologic data for the enrolled patients. Missing data were minimized by providing the physician in charge of the patient and the study nurses with summaries of missing data for each patient in each center and requesting that the missing data be completed whenever possible. The clinical charts of patients for whom an episode of infection was mentioned on the eCRF were retrospectively systematically reviewed by 2 of the authors (M.K. and B.G.). Laboratory and adverse toxic events were graded according to National Cancer Institute Common Terminology Criteria (NCICTC) v4.0 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf). Severe infection was defined as an infection requiring hospitalization and/or intravenous antibiotics and/or resulting in death.
Statistical analysis
Characteristics of patients at inclusion are described with mean ± standard deviation (SD) or median (quartiles 1-3 [Q1-3]) for continuous variables and number (%) for categorical variables. Events of interest are presented with 95% confidence intervals (95% CIs), according to binomial distribution. The cumulative incidence of initial response and complete response as well as recurrence rate after an initial response was examined by the Kaplan-Meier method. Comparison of the 2 rituximab regimens involved the log-rank test. Comparison of responders and nonresponders at 12 months involved the Fisher exact test for categorical data and Student t test or Mann-Whitney test for continuous data. Only patients with an available pattern of response at a mean of 12 ± 3 months were included in this comparison. Predictive factors of 12-month response were analyzed on multivariate analysis by a logistic regression model testing all variables with P ≤ .20 on univariate analysis. P ≤ .05 was considered statistically significant.
Results
Between July 2010 and July 2012, 255 patients were screened for eligibility. Seven did not fulfill the inclusion criteria: 1 patient had secondary ITP (lymphoma); in 1 patient, thrombocytopenia was related to aplastic anemia; 3 patients with primary ITP had received rituximab; and 2 patients were children. Thus, 248 adults were included.
Patient characteristics
Table 1 summarizes the main patient characteristics. All patients had previously received corticosteroids and/or intravenous immunoglobulin (Ig) as first-line treatment of ITP. Only 25 (10%) patients underwent splenectomy. When receiving a first rituximab infusion, 102 patients (41%) had persistent ITP and 146 (59%) chronic ITP. A total of 173 patients (70%) received the “standard regimen” (4 weekly infusions of 375 mg/m2) and 72 (29%) received an “RA-like” regimen (fixed 1-g doses of rituximab on days 1 and 15). Three patients (1%) received only a 1-infusion regimen.
Characteristics of adult patients with ITP treated with rituximab in the “ITP-ritux registry” in France
| Patient characteristics . | . |
|---|---|
| Sex (F/M) | 159/89 (F: 64%) |
| Age (y) at ITP diagnosis, mean ± SD | 51 ± 20 |
| Duration of ITP (months), median (Q1-3) | 16 (5-72) |
| <1 y | 102 (41%) |
| ≥1 y | 146 (59%) |
| Platelet count at ITP diagnosis (×109/L), median (Q1-3) | 18 (6-41) |
| Lower platelet count in the month preceding first rituximab infusion (×109/L), median (Q1-3) | 17 (7-25) |
| Bleeding signs on ITP diagnosis | |
| None | 139 (56%) |
| Bleeding | 109 (44%) |
| No. of previous treatments for ITP, median (Q1-3) | 3 (2-4) |
| Previous treatment | |
| Steroids | 240 (97%) |
| IVIG | 175 (71%) |
| Splenectomy | 25 (10%) |
| Rituximab regimens | |
| 4 weekly infusions (4 × 375 mg/m2) | 173 (70%) |
| 2 fixed 1-g infusions (2 wk apart) | 72 (29%) |
| Other | 3 (1%) |
| Premedication with methylprednisolone | 233 (94%) |
| Dose of methylprednisolone (mg), median (Q1-3) | 63 (40-100) |
| Follow-up duration (mo), median (Q1-3) | 24 (12-30) |
| No. of patients with follow-up >12 mo | 230 (93%) |
| Patient characteristics . | . |
|---|---|
| Sex (F/M) | 159/89 (F: 64%) |
| Age (y) at ITP diagnosis, mean ± SD | 51 ± 20 |
| Duration of ITP (months), median (Q1-3) | 16 (5-72) |
| <1 y | 102 (41%) |
| ≥1 y | 146 (59%) |
| Platelet count at ITP diagnosis (×109/L), median (Q1-3) | 18 (6-41) |
| Lower platelet count in the month preceding first rituximab infusion (×109/L), median (Q1-3) | 17 (7-25) |
| Bleeding signs on ITP diagnosis | |
| None | 139 (56%) |
| Bleeding | 109 (44%) |
| No. of previous treatments for ITP, median (Q1-3) | 3 (2-4) |
| Previous treatment | |
| Steroids | 240 (97%) |
| IVIG | 175 (71%) |
| Splenectomy | 25 (10%) |
| Rituximab regimens | |
| 4 weekly infusions (4 × 375 mg/m2) | 173 (70%) |
| 2 fixed 1-g infusions (2 wk apart) | 72 (29%) |
| Other | 3 (1%) |
| Premedication with methylprednisolone | 233 (94%) |
| Dose of methylprednisolone (mg), median (Q1-3) | 63 (40-100) |
| Follow-up duration (mo), median (Q1-3) | 24 (12-30) |
| No. of patients with follow-up >12 mo | 230 (93%) |
F, female; IVIG, intravenous immunoglobulins; M, male.
At the time of analysis (July 2013), the median follow-up duration was 24 months (Q1-3, 12-30 months), with a follow-up of at least 12 months for 230 patients (93%). Only 2 patients were lost to follow-up just after rituximab infusions; they were considered nonresponders. Sixteen patients had a follow-up between 6 and 12 months.
Safety
In total, 87 adverse events were reported in 44 patients (19%), 66 of which were possibly rituximab-related. Intolerance to rituximab infusions was the most frequent adverse event, with 49 events observed in 38 patients (15%, 95% CI, 11-20). All adverse events related to rituximab infusion were of grade 1 according to the NCICTC, except 3 events of grade 3, which required interruption of treatment because of severe hypotension, dyspnea with laryngeal discomfort, and reversible serum sickness. Infusion-related adverse reactions occurred mainly at the first infusion (n = 30), but some were observed during or after the second (n = 16) or third infusion (n = 3). Rituximab infusions had to be stopped in only 3 patients (as described previously). The most frequent other adverse events related to infusions were minor and transient and included skin rash (n = 11), digestive discomfort (n = 9), transient chills and fever (n = 8), minor laryngeal discomfort (n = 6), paresthesia (n = 4), headache (n = 3), and hypertension and tachycardia (n = 3).
We observed 11 cases of infection in 7 patients (3%; 95% CI, 1-6) (Table 2), corresponding to an incidence of 2.3 infections/100 patient-years (95% CI, 1.2-4.1). We did not observe any case of progressive multifocal encephalopathy or any other opportunistic infections except 1 case of transient aspergillosis sinusitis. Infections occurred 2 to 18 months after the first rituximab infusion. Eight cases recovered, but 3 patients died of infection 12 to 14 months after the first rituximab infusion. These patients were older than 70 years, 2 had severe comorbidities (diabetes and peritoneal carcinosis), and they had received prolonged treatment with corticosteroids for refractory ITP.
Infectious complications after rituximab therapy for ITP
| Sex, age (y) . | Duration of ITP (mo) . | Splenectomy before rituximab treatment . | Site of infection . | Infectious agents . | Delay after first rituximab infusion, months . | Admission to hospital/parenteral antibiotics . | Current treatment with steroids . | Last gammaglobulin level (g/L) . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| F, 38 | 16 | No | Pulmonary | ND | 2 | −/+ | No | NA | Recovery |
| M, 67 | 2 | No | Cutaneous | ND | 2 | −/+ | No | 12.4 | Recovery |
| F, 37 | 2 | No | Pulmonary | Streptococcus pneumoniae | 18 | +/+ | No | 10.2 | Recovery |
| M, 71 | 119 | No | Flu-like syndrome | ND | 4 | −/− | No | NA | Recovery |
| M, 73 | 1 | No | Bone, septic shock | Aureus Staphylococcus | 13 | +/+ | Yes | NA | Death |
| M, 70 | 19 | Yes | Pulmonary | Enterococcus faecium | 12 | +/+ | Yes | 5.4 | Death |
| F, 74 | 2 | No | Urinary tract | Escherichia coli | 1 | +/+ | Yes | 6.2 | Recovery |
| Sinus | Aspergillus fumigatus | 2 | −/+ | Yes | 6.2 | Recovery | |||
| Pulmonary | ND | 2.5 | −/+ | Yes | 6.4 | Recovery | |||
| Urinary tract | Streptococcus, group D | 4 | −/+ | Yes | NA | Recovery | |||
| Septic shock | Enterobacter cloacae | 14 | +/+ | Yes | NA | Death |
| Sex, age (y) . | Duration of ITP (mo) . | Splenectomy before rituximab treatment . | Site of infection . | Infectious agents . | Delay after first rituximab infusion, months . | Admission to hospital/parenteral antibiotics . | Current treatment with steroids . | Last gammaglobulin level (g/L) . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| F, 38 | 16 | No | Pulmonary | ND | 2 | −/+ | No | NA | Recovery |
| M, 67 | 2 | No | Cutaneous | ND | 2 | −/+ | No | 12.4 | Recovery |
| F, 37 | 2 | No | Pulmonary | Streptococcus pneumoniae | 18 | +/+ | No | 10.2 | Recovery |
| M, 71 | 119 | No | Flu-like syndrome | ND | 4 | −/− | No | NA | Recovery |
| M, 73 | 1 | No | Bone, septic shock | Aureus Staphylococcus | 13 | +/+ | Yes | NA | Death |
| M, 70 | 19 | Yes | Pulmonary | Enterococcus faecium | 12 | +/+ | Yes | 5.4 | Death |
| F, 74 | 2 | No | Urinary tract | Escherichia coli | 1 | +/+ | Yes | 6.2 | Recovery |
| Sinus | Aspergillus fumigatus | 2 | −/+ | Yes | 6.2 | Recovery | |||
| Pulmonary | ND | 2.5 | −/+ | Yes | 6.4 | Recovery | |||
| Urinary tract | Streptococcus, group D | 4 | −/+ | Yes | NA | Recovery | |||
| Septic shock | Enterobacter cloacae | 14 | +/+ | Yes | NA | Death |
NA, not available; ND, not determined.
Gammaglobulin levels were not systematically monitored by all centers participating in the study. From the available data provided in Table 3, we observed no substantial decrease in gammaglobulin level during follow-up nor severe hypogammaglobulinemia, except in 1 patient showing hypogammaglobulinemia <5 g/dL without infection at 24 months after rituximab infusions.
Gammaglobulin level after rituximab therapy for ITP
| Date . | No. of tested patients . | Gammaglobulin level (g/dL), median (Q1-3) . | No. of patients with gammaglobulin level ≥5 and <8 g/dL . | No. of patients with gammaglobulin level <5 g/dL . |
|---|---|---|---|---|
| Month 2 | 44 | 10.21 (6.2-19.8) | 9 | 0 |
| Month 4 | 45 | 9.96 (5.7-21) | 11 | 0 |
| Month 6 | 37 | 9.61 (5.4-20.2) | 13 | 0 |
| Month 12 | 43 | 10.09 (5.49-25.8) | 6 | 0 |
| Month 18 | 29 | 11.01 (7.2-17.2) | 2 | 0 |
| Month 24 | 30 | 11.78 (3.66-39.7) | 6 | 1 |
| Date . | No. of tested patients . | Gammaglobulin level (g/dL), median (Q1-3) . | No. of patients with gammaglobulin level ≥5 and <8 g/dL . | No. of patients with gammaglobulin level <5 g/dL . |
|---|---|---|---|---|
| Month 2 | 44 | 10.21 (6.2-19.8) | 9 | 0 |
| Month 4 | 45 | 9.96 (5.7-21) | 11 | 0 |
| Month 6 | 37 | 9.61 (5.4-20.2) | 13 | 0 |
| Month 12 | 43 | 10.09 (5.49-25.8) | 6 | 0 |
| Month 18 | 29 | 11.01 (7.2-17.2) | 2 | 0 |
| Month 24 | 30 | 11.78 (3.66-39.7) | 6 | 1 |
Apart from severe infections, 3 patients experienced a late nonfatal severe adverse event of grade 3 according to the NCICTC, possibly related to rituximab, requiring admission to the hospital. At 7 months after rituximab infusion, 1 patient experienced an episode of profound but transient (7 days) and asymptomatic neutropenia. He spontaneously recovered. One patient experienced transient heart failure at 18 months after rituximab infusion and in 1 patient, inflammatory demyelinating polyneuropathy developed 4 months after a new course of rituximab infusions administered 1 year after the first one.
In all, 13 patients (5%) died during follow-up, at 2 to 39 months after rituximab infusions (Table 4). Two deaths were related to massive bleeding from refractory ITP. Three patients previously described died of infection. A 43-year-old patient died suddenly at home 26 months after rituximab infusions. One 80-year-old patient died at home from an unexplained cause 2 months after rituximab infusions. We cannot exclude that this last death could be rituximab-related. In total, 6 older adults, from 76 to 86 years of age, died 6 to 39 months after rituximab infusions, but rituximab was not implicated.
Causes of death (13 patients) for patients receiving rituximab for ITP
| Sex (F, M)/age (y) . | Cause of death . | Delay after first rituximab infusion (mo) . | Death related to rituximab . |
|---|---|---|---|
| F, 18 | Cerebral hemorrhage, refractory ITP | 2 | No |
| M, 67 | Massive bleeding with shock, refractory ITP | 12 | No |
| F, 43 | Sudden death at home | 26 | No |
| M, 86 | “Old age” | 16 | No |
| M, 97 | “Old age” | 6 | No |
| M, 81 | Cachexia | 16 | No |
| M, 80 | Suicide | 7 | No |
| M, 76 | Multiple myeloma | 13 | No |
| M, 80 | ND | 2 | Possible |
| M, 80 | ND | 39 | No |
| M, 70 | Infection, Enterococcus faecium | 12 | Possible |
| M, 73 | Infection, Staphylococcus aureus | 13 | Possible |
| F, 74 | Infection, Enterobacter cloacae | 14 | Possible |
| Sex (F, M)/age (y) . | Cause of death . | Delay after first rituximab infusion (mo) . | Death related to rituximab . |
|---|---|---|---|
| F, 18 | Cerebral hemorrhage, refractory ITP | 2 | No |
| M, 67 | Massive bleeding with shock, refractory ITP | 12 | No |
| F, 43 | Sudden death at home | 26 | No |
| M, 86 | “Old age” | 16 | No |
| M, 97 | “Old age” | 6 | No |
| M, 81 | Cachexia | 16 | No |
| M, 80 | Suicide | 7 | No |
| M, 76 | Multiple myeloma | 13 | No |
| M, 80 | ND | 2 | Possible |
| M, 80 | ND | 39 | No |
| M, 70 | Infection, Enterococcus faecium | 12 | Possible |
| M, 73 | Infection, Staphylococcus aureus | 13 | Possible |
| F, 74 | Infection, Enterobacter cloacae | 14 | Possible |
Efficacy
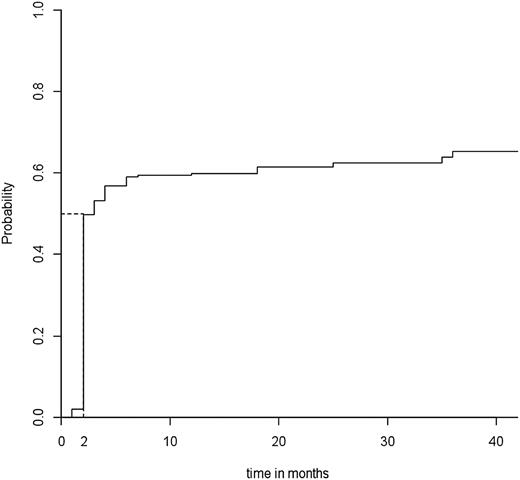
In all, 152 patients (61%) showed an overall initial response at a median time to response of 2 months (Figure 1). The 19 patients without response eventually underwent splenectomy within 1 year after rituximab infusions. After a median follow-up of 24 months (Q1-3, 12-36), 96 patients (39%) showed a lasting response, including 76 with complete response and 20 with response. Among the 152 initial responders, the median follow-up after the date of the response was 25 months. The median time to relapse was 25 months (Figure 2).
Median time to overall response. Median time to overall response in the 248 adult patients in the “ITP-ritux registry.”
Median time to overall response. Median time to overall response in the 248 adult patients in the “ITP-ritux registry.”
Median time to relapse. Median time to relapse among the 152 initial responders to rituximab therapy.
Median time to relapse. Median time to relapse among the 152 initial responders to rituximab therapy.
We analyzed predictive factors of response at 1 year for the 213 patients with an available pattern of response at a mean of 12 ± 3 months after infusions (Table 5). On univariate analysis, response was associated but not significantly with young age, ITP duration <1 year at the time of rituximab infusion, and a previous transient complete response to corticosteroids before being treated with rituximab. The magnitude of response to rituximab did not affect response at 1 year because the proportion of sustained response was similar for the 51 patients with rituximab-related platelet count increase >30 and <100 × 109/L, for the 21 patients with platelet count increase >100 × 109/L, and for the 55 patients with platelet count increase >150 × 109/L with respective percentage of response at 1 year of 30/51 (59%), 13/21 (62%), and 42/55 (76%) (P = .14). On multivariate analysis, response was significantly associated with ITP duration <1 year (odds ratio [OR] 2.02 (95% CI, 1.109-3.690) P = .02]), a previous transient complete response to corticosteroids (OR 2.39 [1.002-5.712], P = .05), and, although not significant, young age (OR 1.01 [0.995-1.002], P = .2).
Possible predictors of response at 1 y after rituximab therapy for ITP
| Variables . | Failure at 1 y (N = 124) . | Response at 1 y (N = 89) . | OR (95% CI) . | P value . |
|---|---|---|---|---|
| Age (y), mean ± SD | 54 ± 20 | 49 ± 20 | 1.012 (0.998-1.026) | .10 |
| Sex (M/F) | 77/47 | 59/30 | 0.833 (0.471-1.473) | .53 |
| ITP duration <1 y before rituximab infusion (n = 87/213) | 44 (36%) | 43 (49%) | 1.715 (0.982-2.994) | .06 |
| Bleeding at ITP diagnosis (n = 90) | 51 (41%) | 39 (44%) | 1.116 (0.644-1.936) | .69 |
| No. of previous treatment lines, median (Q1–3) | 3 (2-4) | 3 (2-4) | 0.944 (0.774-1.151) | .57 |
| Previous splenectomy before rituximab infusion (n = 22) | 15 (12%) | 7 (8%) | 0.62 (0.242-1.591) | .32 |
| Previous transient CR to steroids (n = 46) | 23 (19%) | 23 (26%) | 2 (0.891-4.49) | .09 |
| Previous transient response (R and CR) to IVIG (n = 110) | 65 (71%) | 45 (75%) | 1.2 (0.572-2.516) | .63 |
| Previous transient CR to IVIG (n = 33) | 17 (19%) | 16 (27%) | 1.631 (0.642-4.146) | .30 |
| Platelet count at ITP diagnosis (×109/L), median (Q1-3) | 20 (7-44) | 14 (5-36) | 0.993 (0.981-1.005) | .27 |
| Lower platelet count in the month preceding first rituximab infusion (×109/L), median (Q1-3) | 17 (8-25) | 14 (7-24) | 1.005 (0.992-1.019) | .43 |
| No. of patients with platelet count <10 × 109/L in the month preceding the first rituximab infusion | 31/113* (27%) | 32/82* (39%) | 0.591 (0.322-1.083) | .09 |
| Rituximab infusion regimen | ||||
| 4 infusions | 89 (72%) | 59 (66%) | 0.805 (0.442-1465) | .478 |
| 2 infusions | 34 (28%) | 28 (31%) |
| Variables . | Failure at 1 y (N = 124) . | Response at 1 y (N = 89) . | OR (95% CI) . | P value . |
|---|---|---|---|---|
| Age (y), mean ± SD | 54 ± 20 | 49 ± 20 | 1.012 (0.998-1.026) | .10 |
| Sex (M/F) | 77/47 | 59/30 | 0.833 (0.471-1.473) | .53 |
| ITP duration <1 y before rituximab infusion (n = 87/213) | 44 (36%) | 43 (49%) | 1.715 (0.982-2.994) | .06 |
| Bleeding at ITP diagnosis (n = 90) | 51 (41%) | 39 (44%) | 1.116 (0.644-1.936) | .69 |
| No. of previous treatment lines, median (Q1–3) | 3 (2-4) | 3 (2-4) | 0.944 (0.774-1.151) | .57 |
| Previous splenectomy before rituximab infusion (n = 22) | 15 (12%) | 7 (8%) | 0.62 (0.242-1.591) | .32 |
| Previous transient CR to steroids (n = 46) | 23 (19%) | 23 (26%) | 2 (0.891-4.49) | .09 |
| Previous transient response (R and CR) to IVIG (n = 110) | 65 (71%) | 45 (75%) | 1.2 (0.572-2.516) | .63 |
| Previous transient CR to IVIG (n = 33) | 17 (19%) | 16 (27%) | 1.631 (0.642-4.146) | .30 |
| Platelet count at ITP diagnosis (×109/L), median (Q1-3) | 20 (7-44) | 14 (5-36) | 0.993 (0.981-1.005) | .27 |
| Lower platelet count in the month preceding first rituximab infusion (×109/L), median (Q1-3) | 17 (8-25) | 14 (7-24) | 1.005 (0.992-1.019) | .43 |
| No. of patients with platelet count <10 × 109/L in the month preceding the first rituximab infusion | 31/113* (27%) | 32/82* (39%) | 0.591 (0.322-1.083) | .09 |
| Rituximab infusion regimen | ||||
| 4 infusions | 89 (72%) | 59 (66%) | 0.805 (0.442-1465) | .478 |
| 2 infusions | 34 (28%) | 28 (31%) |
CR, complete response; R, response.
Data are missing for 11 of 124 patients with failure at 1 y and 7 of 89 patients with response at 1 y.
Comparison between 2 rituximab regimens
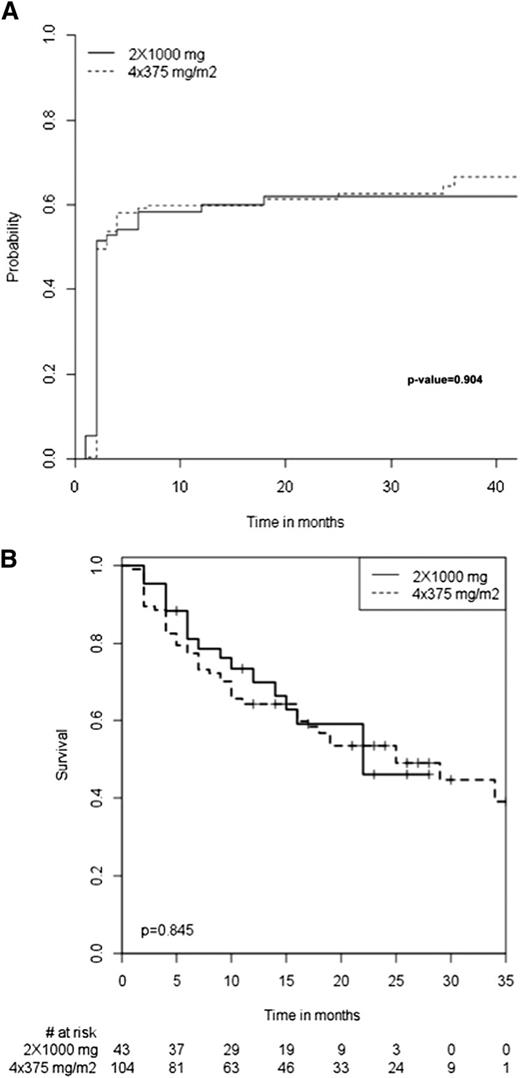
The baseline characteristics of the 173 patients who received the standard regimen (ie, 4 infusions of 375 mg/m2) and the 72 who received the “RA-like” regimen (ie, 2 fixed 1-g infusions 2 weeks apart) were similar except for mean number of previous treatment lines, which was higher for the standard regimen group (Table 6). The mean follow-up duration was also similar between the groups. The immediate tolerance of rituximab infusion was similar between the groups, with an incidence of adverse events of 12.5% for the RA group vs 15.6% for the standard regimen group. All 3 patients for whom rituximab infusions had to be stopped because of severe immediate adverse reactions (described previously) received the standard regimen. The overall initial response and long-term response (Table 6) as well as median time to response (Figure 3A) and to relapse (Figure 3B) for the initial responders were similar between the groups.
Comparison of patients with the 2 rituximab regimens for ITP
| Characteristics . | 2 fixed 1-g infusions (n = 72) . | 4 infusions, 375 mg/m2 (n = 173) . | P value . |
|---|---|---|---|
| Age (y), mean ± SD | 53 ± 20 | 51 ± 21 | .49 |
| Sex (M/F) | 25/47 | 63/110 | .8 |
| Weight (kg), mean ± SD | 74.7 ± 22.2 | 75.0 ± 20.4 | .91 |
| ITP duration | |||
| <1 y | 24 (34%) | 75 (43%) | .16 |
| ≥1 y | 47 (66%) | 97 (56%) | |
| No. of previous treatment lines, mean ± SD | 3.11 ± 1.47 | 2.66 ± 1.29 | .03 |
| Platelet count at ITP diagnosis (×109/L), mean ± SD | 29 ± 28 | 26 ± 24 | .468 |
| Lower platelet count in the month preceding the first rituximab infusion (×109/L), mean ± SD | 22 ± 26 | 18 ± 18 | .23 |
| Follow-up duration (months), mean ± SD | 22 ± 10 | 24 ± 11 | .15 |
| No. with initial response | 44 (61%) | 106 (62%) | .9 |
| No. with long-term response | 29 (40%) | 68 (39%) | .7 |
| Characteristics . | 2 fixed 1-g infusions (n = 72) . | 4 infusions, 375 mg/m2 (n = 173) . | P value . |
|---|---|---|---|
| Age (y), mean ± SD | 53 ± 20 | 51 ± 21 | .49 |
| Sex (M/F) | 25/47 | 63/110 | .8 |
| Weight (kg), mean ± SD | 74.7 ± 22.2 | 75.0 ± 20.4 | .91 |
| ITP duration | |||
| <1 y | 24 (34%) | 75 (43%) | .16 |
| ≥1 y | 47 (66%) | 97 (56%) | |
| No. of previous treatment lines, mean ± SD | 3.11 ± 1.47 | 2.66 ± 1.29 | .03 |
| Platelet count at ITP diagnosis (×109/L), mean ± SD | 29 ± 28 | 26 ± 24 | .468 |
| Lower platelet count in the month preceding the first rituximab infusion (×109/L), mean ± SD | 22 ± 26 | 18 ± 18 | .23 |
| Follow-up duration (months), mean ± SD | 22 ± 10 | 24 ± 11 | .15 |
| No. with initial response | 44 (61%) | 106 (62%) | .9 |
| No. with long-term response | 29 (40%) | 68 (39%) | .7 |
Median times. Median time to response (A) and to relapse (B) among the initial responders to the 2 rituximab regimens.
Median times. Median time to response (A) and to relapse (B) among the initial responders to the 2 rituximab regimens.
Retreatment
In total, 11 patients received retreatment 12 to 42 months after the first course of rituximab; 9 patients received 2 courses of rituximab, 1 patient received 3 courses, and 1 patient received 4 courses (Table 7). A new course of rituximab allowed for a more prolonged response in 3 patients (patients 1, 2, and 3; Table 7). It gave a similar response to the first course in 2 patients (patients 4 and 5) and a worse response in 1 patient (patient 6). The response could not be evaluable in 2 patients (patients 7 and 8). One patient (patient 7) was lost to follow-up immediately after the new course of rituximab. In the other (patient 8), rituximab was associated with a thrombopoietin agonist. In 3 patients who were always responders at the time of last follow-up (patients 9 to 11), more prolonged follow-up is required before being able to compare the response of the new course of rituximab with that obtained with the first course.
Results of rituximab retreatment in 11 patients
| Patient no./sex (M/F) age (y) . | ITP duration (y) . | RTX regimen . | Results of first RTX course/duration (mo) . | Date of second RTX course . | Results of second RTX course/duration (mo) . | Date of third RTX course (mo) . | Results of third RTX course/duration (mo) . | Date of fourth RTX course . | Results of fourth RTX course/duration (mo) . |
|---|---|---|---|---|---|---|---|---|---|
| 1/M 53 | 17 | STD | R/6 | Month 12 | Sustained* RC/12 | ||||
| 2/F 80 | 2.3 | STD | CR/6 | Month 12 | CR/16/relapse | Month 30 | Sustained* CR/12 | ||
| 3/F 51 | 9.6 | STD | CR/6 | Month 18 | Sustained* CR/23 | ||||
| 4/F 47 | 9.4 | STD | Failure | Month 18 | Failure | ||||
| 5/F 71 | 3.4 | STD | CR/14 | Month 18 | CR/12/relapse | Month 30 | CR/2/relapse | Month 36 | CR/7/relapse |
| 6/F 56 | 13.2 | RA | CR/24 | Month 36 | R/3/relapse | ||||
| 7/ M 38 | 16.9 | RA | R/12 | Month 24 | Not evaluable† | ||||
| 8/F 33 | 4.7 | RA | CR/23 | Month 42 | Not evaluable‡ | ||||
| 9/F 45 | 0.17 | STD | CR/13 | Month 24 | Sustained* CR/9 | ||||
| 10/F 65 | 0.9 | RA | CR/23 | Month 23 | Sustained* CR/4 | ||||
| 11/M 37 | 23.9 | STD | CR/36 | Month 42 | Sustained* CR/2 |
| Patient no./sex (M/F) age (y) . | ITP duration (y) . | RTX regimen . | Results of first RTX course/duration (mo) . | Date of second RTX course . | Results of second RTX course/duration (mo) . | Date of third RTX course (mo) . | Results of third RTX course/duration (mo) . | Date of fourth RTX course . | Results of fourth RTX course/duration (mo) . |
|---|---|---|---|---|---|---|---|---|---|
| 1/M 53 | 17 | STD | R/6 | Month 12 | Sustained* RC/12 | ||||
| 2/F 80 | 2.3 | STD | CR/6 | Month 12 | CR/16/relapse | Month 30 | Sustained* CR/12 | ||
| 3/F 51 | 9.6 | STD | CR/6 | Month 18 | Sustained* CR/23 | ||||
| 4/F 47 | 9.4 | STD | Failure | Month 18 | Failure | ||||
| 5/F 71 | 3.4 | STD | CR/14 | Month 18 | CR/12/relapse | Month 30 | CR/2/relapse | Month 36 | CR/7/relapse |
| 6/F 56 | 13.2 | RA | CR/24 | Month 36 | R/3/relapse | ||||
| 7/ M 38 | 16.9 | RA | R/12 | Month 24 | Not evaluable† | ||||
| 8/F 33 | 4.7 | RA | CR/23 | Month 42 | Not evaluable‡ | ||||
| 9/F 45 | 0.17 | STD | CR/13 | Month 24 | Sustained* CR/9 | ||||
| 10/F 65 | 0.9 | RA | CR/23 | Month 23 | Sustained* CR/4 | ||||
| 11/M 37 | 23.9 | STD | CR/36 | Month 42 | Sustained* CR/2 |
RA, “rheumatoid arthritis regimen” for rituximab infusions (ie, 2 fixed 1-g infusions 2 wk apart); RTX, rituximab; STD, “standard regimen” for rituximab infusions (ie, 4 weekly infusions of 375 mg/m2).
Patient was always in remission at the last follow-up.
Patient 7 was not evaluable because a new course of rituximab was associated with treatment with a thrombopoietin agonist.
Patient 8 was not evaluable because of being lost to follow-up immediately after rituximab infusions.
Discussion
We report the largest prospective study, based on a multicenter registry, of patients with ITP treated with rituximab. Our main objective was to assess the safety of rituximab in this setting. The high number of patients included (n = 248) and the median follow-up time of 2 years provides for the first time a clear insight into the safety of rituximab for ITP in a cohort of unselected patients. In this study, we focused on severe infections, which by definition result in hospitalization, intravenous antibiotics use, and/or death and can be easily identified. Clinical nurses monitored and reported every 6 months the systematic review of clinical charts at each participating site to limit the source of bias based on clinician reporting only. The central review of charts allowed us to correctly identify and validate severe infections. Moreover, only 2 patients were lost to follow-up immediately after rituximab infusions. The low percentage of lost to follow-up supported the chosen methodology.
The risk of infection appears acceptable, with a cumulative incidence of 2.3 infections per 100 patient-years. This incidence rate was more than twice lower than that observed in a large registry of patients with RA treated with rituximab.15 Most of the infection cases found recovered without sequelae. Three episodes of infections were fatal, but they occurred at least 1 year after rituximab infusions in older adults who concomitantly received prolonged therapy with corticosteroids for refractory ITP. Two also had severe comorbidities. The role of rituximab as a precipitating factor of infection leading to death is possible but questionable. We observed only 1 episode of opportunistic infection (aspergillosis sinusitis, which resolved) and particularly no PML. The risk of PML can be considered exceptional; to our knowledge, only 2 cases have been reported in ITP patients.13,22
A low IgG level is a well-recognized risk factor for infection, as commonly observed in diseases such as common variable immunodeficiency or chronic lymphocytic leukemia-associated hypogammaglobulinemia. In children, rituximab can induce severe secondary hypogammaglobulinemia, and some authors have recommended the transient use of Ig replacement therapy after rituximab administration, especially in children younger than 5 years.23-25 In adults, the risk of hypogammaglobulinemia after rituximab is much lower, although not clearly established, and no data support prophylaxis for patients with normal baseline Ig levels. This situation explains why these data were frequently missing in our study. Nonetheless, we observed only 1 case of severe hypogammaglobulinemia and no patients had a history of recurrent infections of the upper respiratory tract that could evoke secondary hypogammaglobulinemia. More data with a prolonged follow-up are required, and rare cases of late-onset rituximab-induced hypogammaglobulinemia cannot be excluded.
Rituximab was administered rather early in the course of the disease for most patients mainly to avoid or postpone splenectomy:26 half had persistent ITP; they received a median of 3 therapeutic lines before receiving rituximab, and did not undergo splenectomy. These observations could explain the relatively low incidence of severe infections and we cannot exclude that this risk could be higher in patients receiving prolonged treatment with corticosteroids and/or immunosuppressors.
Fatal infusion-related reactions have been reported in patients receiving rituximab for RA and in other situations; health authorities recently recommended systematic premedication with 100 mg methylprednisolone.27,28 Most of our patients received such premedication, which may explain why rituximab infusions were well tolerated and no severe infusion-related reactions were observed.
Among the rare serious adverse events requiring admission to hospital, late-onset transient and asymptomatic neutropenia developed in only 1 patient. Late-onset neutropenia is a well-known side effect of rituximab mainly reported in the setting of lymphoma, with an estimated incidence of 7%.29,30 This risk could be related to the cumulative dose of rituximab and is likely increased with concomitant chemotherapy.29 These observations may explain why late-onset neutropenia resulting from rituximab appears exceptional in ITP.
Two meta-analyses that pooled data for 313 and 368 patients with and without splenectomy reported an overall response rate to rituximab for ITP of about 60%.14,31 Our prospective study, which included the largest number of patients, confirms that the overall response rate is good and agrees with previous results. We found a lower long-term response (about 40%), with relapse in 40% of the initial responders after 2 years of follow-up. However, the median time to relapse was longer than 2 years, which allowed for observing most responders off-treatment of a prolonged period. A collaborative multicenter retrospective study recently reported a response rate of only 20% in adults with ITP after 5 years of follow-up.16 The 5-year follow-up planned for this registry will provide new insights into the long-term response to rituximab in ITP.
In view of the relatively low rate of long-term response, new strategies to improve the long-term efficacy of rituximab or other B-cell targeting therapies should be developed. Moreover, identifying patients likely to achieve a lasting response with rituximab is still an unmet medical need. Our study confirms that the probability of sustained response is high when rituximab is administered early during the course of ITP, with a possible disease-modifying effect as previously suggested by Stasi et al.32 However, in the absence of a control group, the greater probability of sustained response observed in the patients with persistent ITP could be explained by the natural history of ITP with the possibility of spontaneous remission in this setting.9 Response may be high for young patients, as we previously reported.26 Our study shows for the first time that a previous complete response to corticosteroids is the best predictor of sustained response to rituximab. Unfortunately, none of our predictors of response had sufficient impact for clinical practice in deciding to treat or not on an individual basis.
Most studies of rituximab used for ITP tested the “standard regimen” used in lymphoma (4 weekly infusions of 375 mg/m2).7 A retrospective study we conducted previously suggested that the “RA-like” regimen could be as effective as the standard regimen in ITP.19 The results from our prospective registry including higher number of patients confirm these preliminary data. Up to one-third of our patients received the RA-like regimen. The pattern and magnitude of short- and long-term response and the safety profile were similar with both regimens. Because our study was not randomized, we cannot exclude a bias, but it should be underlined that the characteristics of the 2 groups were similar, except for the number of previous therapeutic lines, which was higher in the group receiving the 2-infusion regimen. Thus, the similar efficacy of the 2 regimens would not be due to recruitment bias. Our results highly suggest that the “RA-like” regimen may be a good alternative for treating ITP.
Little is known about the efficacy of repeated courses of rituximab in patients with relapse after an initial response. A retrospective study by Hasan et al33 showed that in many patients, retreatment induces a similar response, and neither combining rituximab with cyclophosphamide, vincristine, and prednisone nor doubling the dose increases the response rate. Our study confirmed that a similar pattern of response is frequently observed, but of note, a more prolonged response was observed in about one-third of patients. The number of retreated patients was too low for definite conclusions, and a prospective study including more patients should be conducted to better evaluate the efficacy and safety of repeated courses of rituximab.
The limitations of the study include missing data and the relatively short follow-up. Some of the missing data and particularly an inhomogeneous gammaglobulin-level survey were related to the observational nature of the study and reflect current real-life practice. The follow-up duration is currently limited to a median of 2 years and does not allow for analyzing long-term safety and efficacy of rituximab, and the incidence of possible late adverse events such as hypogammaglobulinemia remains unknown.
To conclude, the findings of this large prospective registry of patients with ITP treated with rituximab in “real life” show that the safety profile is acceptable and confirm that the treatment leads to an overall response rate of 39% at 2 years. With its benefit/risk ratio, rituximab used off-label may remain a valid option, particularly for young patients with persistent ITP and a previous transient complete response to corticosteroids because this subgroup has a high probability of sustained response.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Laura Smales for editorial assistance; S. Baloul and L. Languille for their help for the monitoring of the study and for technical assistance, and all the others physicians who had patients in this study: M. Karhaman, A.S. Morin, J. Pelegrini, J.M. Michot, G. Maigne, D. Bordessoule, S. Girault, M.P. Gourin-Chaury, X. Delbrel, D. Bouscary, B. Pegourie, J.Y. Cahn, N. Schleinitz, A. Ghali, D. Verrot, M. Hamidou, A. Masseau, A. Delmer, A. Perlat, D. Adoue, J. Magnant, A.L. Buchdahl, B. Royer, S. Rosenstingl, and E. Rosenthal.
This study was initiated by the investigators and partially financed with an open grant from Roche France, which played no role in designing the study, collecting and analyzing the data, or writing the article.
Authorship
Contribution: B.G., M.K., F.R.T., P.B., and M.M. designed the study and initiated this work; M.K. and B.G. obtained data; B.G., M.K., M.M., and F.R.T wrote the report; and B.G., A.C.N., and F.R.-T. performed all statistical analyses.
Conflict-of-interest disclosure: M.K. and M.M. served on Scientific Advisory Boards for Roche, GSK, and Amgen Laboratories. B.G. is a consultant for Amgen, GSK, and Roche France, received research funds from Roche France, and serves on the Advisory Board for GlaxoSmithKline. G.S. declared consultancy, honoraria, and/or advisory board membership from Roche and/or Genentech. The remaining authors declare no competing financial interests.
Correspondence: Bertrand Godeau, Department of Internal Medicine, Centre Hospitalier Universitaire Henri-Mondor, 51, Avenue du Maréchal-de-Lattre-de-Tassigny, 94010 Créteil Cedex, France; e-mail: bertrand.godeau@hmn.aphp.fr.