Abstract
Background
Patients with cancer and VTE have a substantial risk of recurrent VTE. LMWH reduces the risk of symptomatic, recurrent VTE compared with warfarin and is recommended as the preferred anticoagulant by consensus guidelines. However, the evidence is based largely on a single, open-label randomized trial (CLOT; Lee et al NEJM 2003). Warfarin is still often used for the treatment of VTE in cancer patients worldwide.
Methods
The primary objective of this randomized, open-label, multicenter, Phase III trial (CATCH; NCT01130025) was to assess the efficacy of tinzaparin in preventing recurrent VTE in patients with active cancer and acute, symptomatic proximal deep vein thrombosis (DVT) and/or pulmonary embolism (PE). Patients were randomized (stratified by geographic region, tumor characteristic [distant metastasis, no distant metastasis, hematological malignancy] and history of VTE) to receive tinzaparin 175 IU/kg once daily for 6 months or initial tinzaparin 175 IU/kg once daily for 510 days overlapped and followed by dose-adjusted warfarin (target INR 2.03.0) for 6 months. The primary efficacy outcome was time to recurrent VTE verified by objective, standard imaging and blinded central adjudication; this was a composite primary endpoint that included symptomatic DVT and/or PE, incidental proximal DVT and/or PE and fatal PE. The primary safety endpoint was incidence of major bleeding. All patients were followed up to 6 months or death, whichever came sooner. Blinded central adjudication was also performed for all bleeding events and causes of death. A proportional hazards model for competing risks was applied to all randomized patients, treating all non-VTE-related deaths as competing events. An independent Data Safety Monitoring Board reviewed safety data at regular intervals.
Results
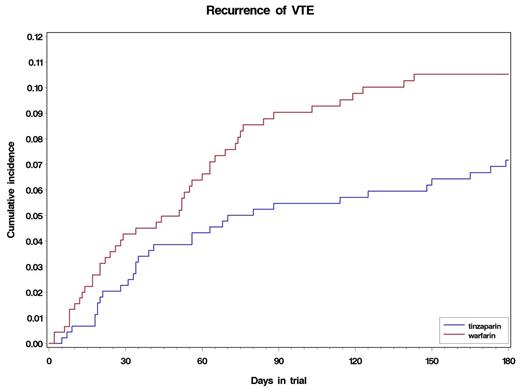
Nine hundred patients were included from 165 sites in 32 countries across 5 continents. Of these, 449 were randomized to tinzaparin and 451 to warfarin. Mean age was 59 years (range 1889); 59% female. A total of 77% of patients had a baseline ECOG performance status (PS) of 01 and 23% had a PS of 2. The most common primary tumor sites were gynecologic (23%), colorectal (13%), lung (12%), breast (9%); 10% had hematological malignancies. At the time of randomization, metastatic disease was present in 55% of patients and 44% had received prior cancer treatment (chemotherapy, surgery and/or radiation). Time-in-therapeutic range was 47% in the warfarin arm, with 27% above and 26% below the range. Over the 6-month trial period, 31 patients (6.9%) in the tinzaparin arm experienced recurrent VTE compared with 45 (10%) in the warfarin arm (hazard ratio [HR] 0.65 [95% CI 0.411.03; P=0.07]) (see figure). There were 2 patients with incidental VTE, both were in the warfarin arm. Symptomatic non-fatal DVT occurred in 12 patients (2.7%) in the tinzaparin arm and 24 (5.3%) in the warfarin arm (HR 0.48 [95% CI 0.240.96]; P=0.04). Symptomatic non-fatal PE occurred in 3 patients in the tinzaparin arm and 2 in the warfarin arm; fatal PE occurred in 17 (3.8%) patients in each arm (HR 0.96 [95% CI 0.491.88]; P=0.90). There was no difference in the incidence of major bleeding events (n=13 [2.9%] in the tinzaparin arm and 12 [2.7%] in the warfarin arm), but significantly fewer patients experienced clinically relevant non-major bleeding with tinzaparin than warfarin (50 [11%] and 73 [16%] patients, respectively; P=0.03). No difference in mortality was seen with 6-month survival rates of 59% and 60%, respectively.
Conclusions
In cancer patients with symptomatic VTE, tinzaparin lowered the risk of recurrent VTE compared with warfarin, with a significant reduction in symptomatic DVT and clinically relevant non-major bleeding. No difference in major bleeding or overall mortality was observed.
Cumulative incidence of recurrent VTE in the tinzaparin and warfarin groups.
Lee:Bayer: Advisory Boards Other, Honoraria; Bristol-Myers Squibb: Advisory Boards, Advisory Boards Other, Research Funding; Boehringer Ingelheim: Honoraria; Daiichi-Sankyo: Advisory Boards, Advisory Boards Other; Eisai: Research Funding; LEO Pharma: Advisory Boards Other; Pfizer: Advisory Boards Other, Honoraria, Research Funding; Sanofi-Aventis: Advisory Boards, Advisory Boards Other; Avivia: Advisory Boards, Advisory Boards Other. Kamphuisen:LEO Pharma: Honoraria, Research Funding. Meyer:Bayer: Research Funding; Boehringer Ingelheim: Research Funding; LEO Pharma: Research Funding; Sanofi-Aventis: Research Funding. Janas:LEO Pharma: Employment. Jarner:LEO Pharma: Employment. Khorana:LEO Pharma: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal