Abstract
Background
Several studies, including retrospective analyses of patient registries1,2 and a subanalysis of the phase III MDS-AZA-001 trial3 suggest that poor-risk cytogenetics negatively impact overall survival (OS) in patients with myelodysplastic syndrome (MDS) and World Health Organization (WHO)-defined acute myeloid leukemia (AML) treated with azacitidine (AZA).
There are few data available to indicate whether AZA has improved clinical activity vs conventional care in AML patients with adverse cytogenetics. However, in a subanalysis of MDS-AZA-001 (MDS and AML [20–30% bone marrow blasts]) patients with –7/–7q abnormalities had better OS with AZA than low-dose cytarabine (21.4 vs 3.5 months, respectively) supporting significant activity of AZA in patients with adverse cytogenetics.4
Methods
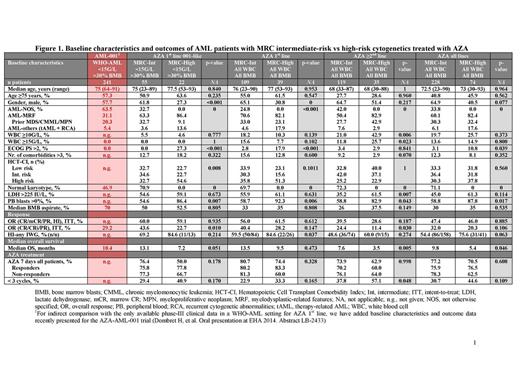
In this retrospective study of the Austrian AZA Registry (N=346), we compared patients with WHO-AML and intermediate- (n=228) vs high-risk (n=74) cytogenetics according to Medical Research Council (MRC) criteria. Outcomes were also assessed with respect to AZA treatment line.
Results
The intermediate-risk cytogenetics group comprised 228 patients (AZA 1st line, n=109; AZA ≥2nd line, n=119), and the high-risk cytogenetics group comprised 74 patients (AZA 1st line, n=39; AZA ≥2nd line, n=35; Figure 1). Comparison of baseline characteristics of both groups revealed significant differences with regard to prevalence of males and Eastern Cooperative Oncology Group Performance Status (ECOG PS) >2 for patients with high-risk cytogenetics receiving AZA 1st line, but not in those receiving AZA ≥2nd line. Peripheral blood blasts were present in a significantly larger proportion of high- than intermediate-risk patients (Figure 1).
In patients who received AZA 1st line, median number of AZA cycles was 6 for both the intermediate- and high-risk cytogenetic groups (range: 1–46 and 1–25, respectively). Median time from diagnosis to AZA start was <1 month for AZA 1st line and >7.6 months for AZA ≥2nd line. Median time from AZA stop to death was <2 months in all cohorts.
In the whole cohort, the overall response rate (ORR) according to International Working Group (IWG) 2003 criteria5 was similar for patients with intermediate- and high-risk cytogenetics (complete response [CR] + CR with incomplete blood count recovery [CRi] + partial response [PR]: 32.0 vs 20.3%; p=0.106; Figure 1). Rates of hematologic improvement (HI) according to IWG 2006 criteria6 were also not significantly different (54.4 vs 75.6; p=0.063), and when ORR and HI were combined, the difference remained non-significant (47.4 vs 46.0%; p=0.885; Figure 1).
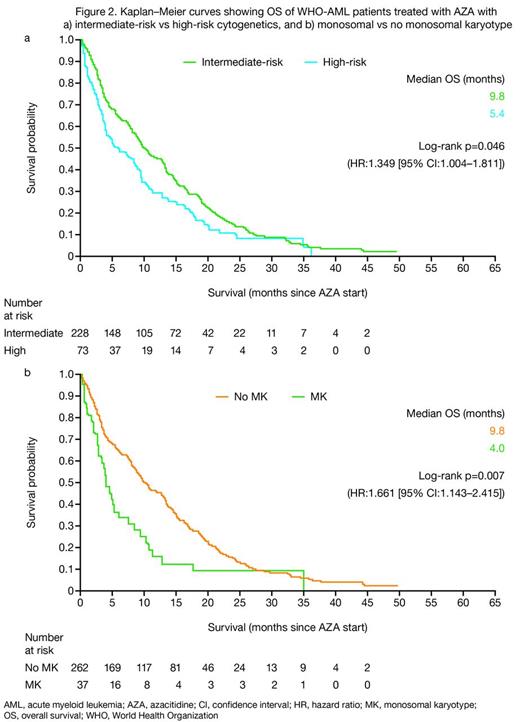
Median OS was consistently higher in patients with intermediate- than high-risk cytogenetics (9.8 vs 5.4 months for the total cohort; p=0.046 [Figures 1 and 2a]; 13.5 vs 9.5 months for AZA 1st line [not significant]; and 7.6 vs 3.5 months for AZA ≥2nd line; p=0.005 [Figure 1]). However, median OS for responding patients (CR/CRi/PR/HI) was similar for patients with intermediate- and high-risk cytogenetics, irrespective of treatment line (19.9 vs 19.3 months for all responders; 20.5 vs 21.7 months for AZA 1st line; and 18.5 vs 15.0 months for AZA ≥2nd line). Furthermore, presence of a monosomal karyotype had a significant negative impact on OS (Figure 2b). None of the baseline factors analyzed had an impact on OS in patient subgroups with intermediate- or high-risk cytogenetics, except number of comorbidities >3.
Conclusions
Here, we compared outcomes of 302 WHO-AML patients with intermediate- vs high-risk cytogenetics treated with AZA. In line with recent data of MDS patients,1 baseline cytogenetics did not seem to have a significant effect on response to AZA. However, in agreement with other studies of AZA in MDS/WHO-AML patients,1–3 high-risk cytogenetics had a negative impact on survival compared with intermediate-risk cytogenetics in WHO-AML treated with AZA.
1. Sebert M, et al. Oral presentation at ASH 2013. Abstract 389
2. Thepot S, et al. Am J Hematol 2014;89:410–6
3. Fenaux P, et al. J Clin Oncol 2010;28:562–9
4. Fenaux P, et al. Br J Haematol 2010;149:244–9
5. Cheson BD, et al. J Clin Oncol 2003;21:4642–9
6. Cheson BD, et al. Blood 2006;108:419–25
Pleyer:AOP Orphan Pharmaceuticals: Honoraria; Novartis: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Off Label Use: Vidaza (azacitidine) is indicated for the treatment of adult AML patients who are not eligible for haematopoietic stem cell transplantation with 20–30 % blasts and multi-lineage dysplasia, according to WHO classification. This cohort also includes AML-patients with >30% bone marrow blasts.. Burgstaller:AOP Orphan Pharmaceuticals: Honoraria; Novartis: Honoraria; Mundipharma: Honoraria; Celgene: Consultancy. Stauder:Novartis: Research Funding; Ratiopharm: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding. Girschikofsky:Pfizer: Honoraria, Research Funding; Mundipharm: Consultancy, Honoraria. Pfeilstöcker:Janssen-Cilag: Honoraria; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Lang:Celgene: Consultancy. Sperr:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Phadia: Research Funding. Valent:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Greil:Sanofi Aventis: Honoraria; Roche: Honoraria; Pfizer: Honoraria, Research Funding; Boehringer-Ingelheim: Honoraria; Astra-Zeneca: Honoraria; Novartis: Honoraria; Genentech: Honoraria, Research Funding; Janssen-Cilag: Honoraria; Merck: Honoraria; Mundipharma: Honoraria, Research Funding; Eisai: Honoraria; Amgen: Honoraria, Research Funding; Celgene: Consultancy, Research Funding; Cephalon: Consultancy, Honoraria, Research Funding; Bristol-Myers-Squibb: Consultancy, Honoraria; GSK: Research Funding; Ratiopharm: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal