Abstract
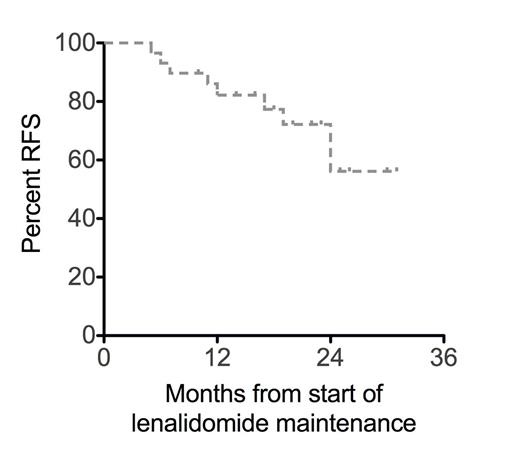
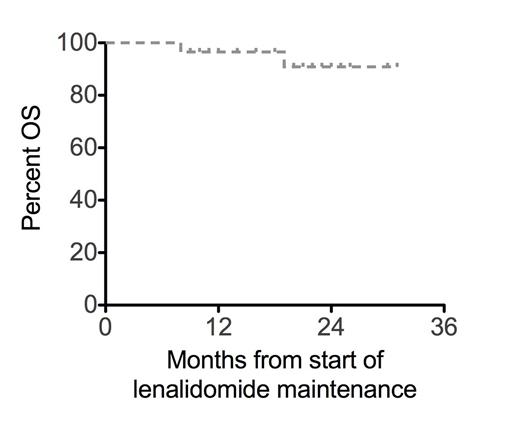
Relapse risk in the first 12 months after achieving complete remission (CR) is approximately 40% in the adult acute myeloid leukemia (AML) population (Bradstock, Blood 2005; Burnett, JCO 2013). In this phase 1b study conducted by the ALLG, patients with AML in first CR or CR with incomplete count recovery (CRi) after intensive chemotherapy were allocated to escalating dose cohorts of lenalidomide maintenance; 10 mg (n=8), 20 mg (n=9), 30 mg (n=9) or 40 mg (n=4) administered daily for two continuous 28-day cycles, followed by lenalidomide 10 mg daily for a total lenalidomide duration of 12 months. To date, 28 patients have been enrolled from 8 centers. The median age is 54 (range 18-65) years, ECOG performance status was 0 in 82% and 1 in 18%. Preceding chemotherapy for patients 18-55 years was HiDAC-3 induction (cytarabine 3 g/m2 bd D1, 3, 5, 7 + idarubicin 12 mg/m2 D1-3) and IcE (idarubicin 12 mg/m2 D1-2, cytarabine 100 mg/m2 D1-5 and etoposide 75 mg/m2 D1-5) consolidation. Patients aged 56-65 years received 7+3 induction (cytarabine 100 mg/m2 CIVI D1-7 + idarubicin 12 mg/m2 D1-3) and IDAC-2 (intermediate dose cytarabine 1 g/m2 bd D1, 3, 5 + idarubicin 12 mg/m2 D1-2) consolidation. Patients with core binding factor subtypes were consolidated with 3-4 cycles of HiDAC (cytarabine 3g/m2 bd D1,3,5). Patients received either one (3%), two (79%) or three-four (18%) cycles of consolidation chemotherapy prior to study entry. Cytogenetic risk at diagnosis was favorable (29%), intermediate (67%) or adverse (4%) by MRC criteria (Grimwade, Blood 2010). Molecular features included FLT3-ITD in 33% and NPMc mutation in 26%. During cycles 1-2 the frequency of grade 3-4 neutropenia/thrombocytopenia for each lenalidomide cohort was: 10 mg (13/13%), 20 mg (11/0%), 30 mg (50/12%), 40 mg (75/25%). Four patients have developed grade 3 skin rash on study. Two patients experienced grade 3 febrile neutropenia but there were no treatment-related deaths. The maximum tolerated dose has not been reached and recruitment is continuing at the 50mg dose level. With a median follow-up of 23 months, actuarial incidence of relapse in the first 12 months after commencing lenalidomide was 18% and 26% in the second year (Figure 1A). Overall survival (OS) after starting lenalidomide maintenance was 97% at 12 and 91% at 24 months (Figure 1B). Relapse-free survival (RFS) 24 months after starting lenalidomide maintenance according to cytogenetic or molecular factors was 78% (favorable karyotype), 48% (intermediate karyotype), 75% (NPMc mutation), 31% (FLT3-ITD) and 53% (FLT3-ITD and NPMc double negative). In conclusion, lenalidomide maintenance in an adult AML population has acceptable tolerance, even after prior HiDAC. Relapse risk in the first year during lenalidomide maintenance is lower than historically observed, but later relapses continue to be seen suggesting that future studies could investigate extended duration schedules of lenalidomide maintenance.
RFS and OS for lenalidomide maintenance therapy in first complete remission
Wei:Celgene: Consultancy, Honoraria, Research Funding. Off Label Use: Lenalidomide as maintenance therapy for AML. Hertzberg:Celgene: Consultancy, Honoraria. Seymour:Celgene: advisory board Other, Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal