Abstract
The regulation of cell volume is important for the maintenance of integrity in all cells and is especially critical for the highly specialized red blood cell (RBC) which must withstand pressure changes within the vasculature and remain deformable to traverse small vessels. Disorders that interfere with volume homeostasis result in the premature destruction of RBCs. One protein that appears to play a prominent role in RBC hydration is the recently described nonselective cation channel PIEZO1 which is involved in mechanotransduction. Mutations of PIEZO1 have been associated with an autosomal dominant form of hereditary hemolytic anemia (HHA) characterized by RBC dehydration known as hereditary xerocytosis (HX) (Zarychanski et al., Blood 2012;120:1908). There is evidence that PIEZO1 may also be responsible for a channel activity that participates in the dehydration of sickle cells which exacerbates sickling and vaso-occlusive events in patients with Sickle Cell Disease (reviewed in Gallagher, Curr Opin Hematol 2013, 20:201).
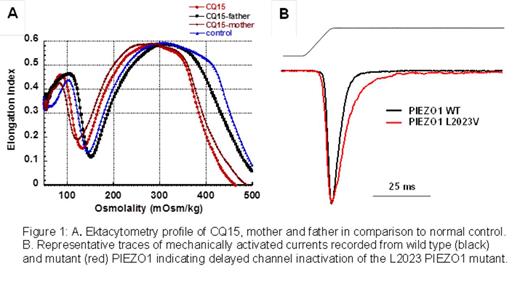
Using a Next-Generation sequencing panel containing 27 hemolytic anemia associated genes, we identified and characterized a novel PIEZO1 mutation p.L2023V which results in delayed channel inactivation and a dehydrated RBC phenotype. This single amino acid substitution at a highly conserved site was detected in the heterozygous state in a 15 year old Caucasian young man (CQ15) with chronic hemolysis well compensated with reticulocytosis, along with heterozygosity for the SLC4A1 p.E40K mutation known as band 3 Montefiore. Hematologic characteristics included macrocytosis, elevated MCHC, and a smear showing occasional stomatocytes. Several family members also had hemolysis and jaundice and were given a diagnosis of hereditary pyropoikilocytosis prior to our evaluation. The L2023V mutation was considered possibly damaging by the PolyPhen-2 algorithm with a score of 0.777. The mother was heterozygous for the PIEZO1 L2023V mutation while the father carried the band 3 Montefiore mutation. Ektacytometry was used to evaluate RBC deformability; the mother's RBCs had a profile of classic xerocytosis with decreased Omin and Ohyp, the father had a very mild spherocytosis profile, while the patient's RBCs had mixed ektacytometry characteristics with decreased Ohyp and an intermediate Omin (Figure 1A). Intracellular cation values determined by flame emission spectroscopy for CQ15 and his mother demonstrated K+ loss. The mutation p.L2023V is located at a site predicted to be at the border between membrane and cytoplasm in the carboxy terminal part of the protein, similar to the previously described p.R2456H mutation in a kindred with HX (Zarychanski et al., 2012).
To enable detailed physiologic study of the L2023V mutation, we prepared an HEK293 cell line with a stable, single copy integrant of the variant with an inducible promoter. Whole cell patch clamp studies were performed on HEK293 cells expressing wild type PIEZO1 and PIEZO1 L2023V. Traces of mechanically activated currents were recorded from the cells and were normalized to peak current (Figure 1B). The inactivation time constant tau (in ms) was determined from mono-exponential fits for wild type and mutant PIEZO1 channels. The difference in average inactivation time between wild type PIEZO1 and PIEZO1 L2023V was highly significant (Student's t-test; p<0.0001) and is predicted to lead to cellular dehydration.
Patients often present with a complex clinical picture and laboratory results and may have combinations of potentially damaging genetic variants identified by Next-Generation sequencing. The examination of clinical, laboratory, and genetic data from family members and, in some cases, in vitro studies are required to clarify the relative contribution of these variants and to arrive at an accurate diagnosis. The data presented here further our understanding of the role of PIEZO1 in RBCs and its potential pathological contribution in HHAs with associated cellular dehydration and may facilitate the future development of therapeutic targets for treatment of these conditions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal