Abstract
Background
The outcome of primary refractory (PRF) acute myeloid leukemia (AML) patients is poor with a minor proportion rescued by allogeneic hematopoietic stem cell transplantation (HSCT). The identification of pre-HSCT variables may help to identify PRF AML most likely to benefit from HSCT. The EBMT group reported factors predicting the outcome of 168 patients with PRF AML receiving an unrelated donor stem cell transplantation; 5-years OS was 22% and factors associated to an improved survival were the numbers of chemotherapy cycles (< 3), bone marrow blast infiltration <38% and patient CMVseropositivity. These clinical findings allowed to define 4 prognostic groups with survival rates ranging between 44% and 0% {Craddock, 2011). We performed a similar analysis focusing on PRF AML patients transplanted in Italy between 1999-2012 with a stem cell graft obtained by a sibling, unrelated donor and cord blood unit.
Patients and study design
We analyzed the clinical outcome of 242 patients transplanted in 26 GITMO centers. Patients disease status at HCST included PRF AML defined as failure to achieve a complete response (CR) after one or more chemotherapy cycles containing active drugs on AML. The cytogenetic and molecular risk was defined according to the European LeukemiaNet. The main clinical and outcome follow up data were retrieved from the GITMO database. The main end-points of the study were overall survival (OS) and leukemia-free survival (LFS).
Results
The median age at HSCT was 49 years (18-72) and 55% of patients were male. Before HSCT, 58% received ≤ 2 chemotherapy cycles. Median time from diagnosis to HSCT was 6 months (1-19) and in 85% was ≥ 3 months. An intermediate-II/adverse karyotype was detected in 58% of patients, > 25% marrow blast infiltration or any level of peripheral blood (PB) blasts was found in 60% and a pre-HSCT Karnofsky score < 90 was present in 43%. Donors were HLA identical sibling in 48% and matched unrelated in 19%, related mismatched in 19%, unrelated mismatched in 3% and cord blood in 11%. Anti-CMV antibodies were present in 87% of patients and in 65% of the donors. Conditioning regimen intensity was myeloablative or reduced in 69% and 31%, respectively; 49% of patients received T cell depletion (92% in vivo and 8% ex vivo). Neutrophils and platelets engraftment was achieved in 87% of patients after a median of 17 (9-52) and 17 (3-150) days, respectively.
In all, 35 (14%) patients died within 30 days from HSCT. Of 207 patients evaluable for response, 138 (66%) achieved CR after a median time of 32 days (range 16-130) from HSCT and 69 did not (33%). Median survival of patient who achieved CR was 10 months while it was 2 months for those who did not. Seventy patients (51%) relapsed after a median time of 3 months (1-31), 64 died of disease, 6 survived and 2 of these latter reachieved CR. Sixty-eight patients (49%) maintained CR, 34(50%) died and 34 survived. A t the last follow up 42 patients were alive, 36 in CR and 6 with disease with a median follow up of 27 months (range 1,8-14). The median OS of the whole patient cohort was 5,7 months. At 3-years, the OS and LFS was 15% and 23% respectively.
AGvHD was registered in 39% of patients (grade > 2 in 30% of cases) while cGVHD occurred in 29% (extended in 44% of cases). The 3-years cumulative incidence of NRM was 17%.
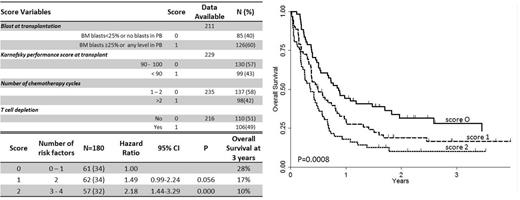
By univariate analysis, the number of chemotherapy cycles to achieve CR (≤ 2), the time to HSCT (< 3 months), the cytogenetics risk favorable/intermediate I, the number of marrow blasts < 25% or the absence of blasts in the peripheral blood, the PS ≥ 90 and the lack of any form of T cell depletion, were all associated to a better survival. By multivariate analysis, the number of chemotherapy cycles, (Hazard Ratio (HR): 1.51; 95% confidence interval (CI): 1.04–2.19; P=0.029), the lack of T cell depletion (HR: 1.66; 95% CI: 1.15–2.40; P=0.007), the degree of BM or PB blast infiltration (HR: 1.59; 95% CI: 1.01–2.25; P=0.043), and the PS (HR: 1.46; 95% CI: 1.00–2.14; P=0.048) remained significantly associated with survival. On the basis of this multivariate analysis, we set up a new score predicting a different 3 years OS: score 0 (0 or 1 adverse prognostic factor, with 28% survival), score 1 (2 adverse prognostic factor, 17% survival); score 2 (2 or 3 adverse prognostic factors, 10% survival) (Figure 1)
Conclusion
The clinical outcome of PRF AML remains poor. The new simple clinical GITMO score helps indentifying patients most likely will benefit or not from the HSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal